| << Chapter < Page | Chapter >> Page > |
We can study many different features of solids. Just a few of the things we could study are how hard or soft they are, what their magnetic properties are or how well they conduct heat. The thing that we are interested in, in this chapter are their electronic properties. Simply, how well do they conduct electricity and how do they do it.
We are only going to discuss materials that form a 3-dimensional lattice. This means that the atoms that make up the material have a regular pattern (carbon, silicon, etc.). We won't discuss materials where the atoms are jumbled together in a irregular way (plastic, glass, rubber etc.).
We know that there are materials that do conduct electricity, called conductors, like the copper wires in the circuits you build. There are also materials that do not conduct electricity, called insulators, like the plastic covering on the copper wires.
Conductors come in two major categories: metals (e.g. copper) and semi-conductors (e.g. silicon). Metals conduct very well and semi-conductors don't. One very interesting difference is that metals conduct less as they become hotter but semi-conductors conduct more.
What is different about these substances that makes them conduct differently? That is what we are about to find out.
We have learnt that electrons in an atom have discrete energy levels. When an electron is given the right amount of energy, it can jump to a higher energy level, while if it loses the right amount of energy it can drop to a lower energy level. The lowest energy level is known as the ground state.
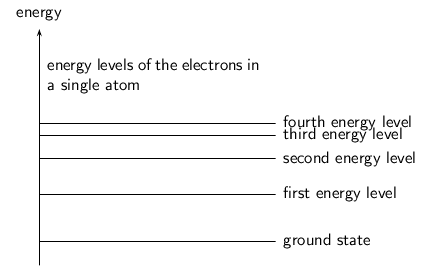
When two atoms are far apart from each other they don't influence each other. Look at the picture below. There are two atoms depicted by the black dots. When they are far apart their electron clouds (the gray clouds) are distinct. The dotted line depicts the distance of the outermost electron energy level that is occupied.
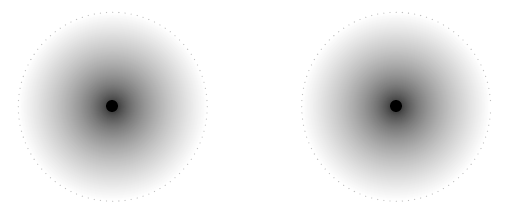
In some lattice structures the atoms would be closer together. If they are close enough their electron clouds, and therefore electron energy levels start to overlap. Look at the picture below. In this picture the two atoms are closer together. The electron clouds now overlap. The overlapping area is coloured in solid gray to make it easier to see.

When this happens we might find two electrons with the same energy and spin in the same space. We know that this is not allowed from the Pauli exclusion principle. Something must change to allow the overlapping to happen. The change is that the energies of the energy levels change a tiny bit so that the electrons are not in exactly the same spin and energy state at the same time.
So if we have 2 atoms then in the overlapping area we will have twice the number of electrons and energy levels but the energy levels from the different atoms will be very very close in energy. If we had 3 atoms then there would be 3 energy levels very close in energy and so on. In a solid there may be very many energy levels that are very close in energy. These groups of energy levels are called bands. The spacing between these bands determines whether the solid is a conductor or an insulator.

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 11 physical science' conversation and receive update notifications?