| << Chapter < Page | Chapter >> Page > |
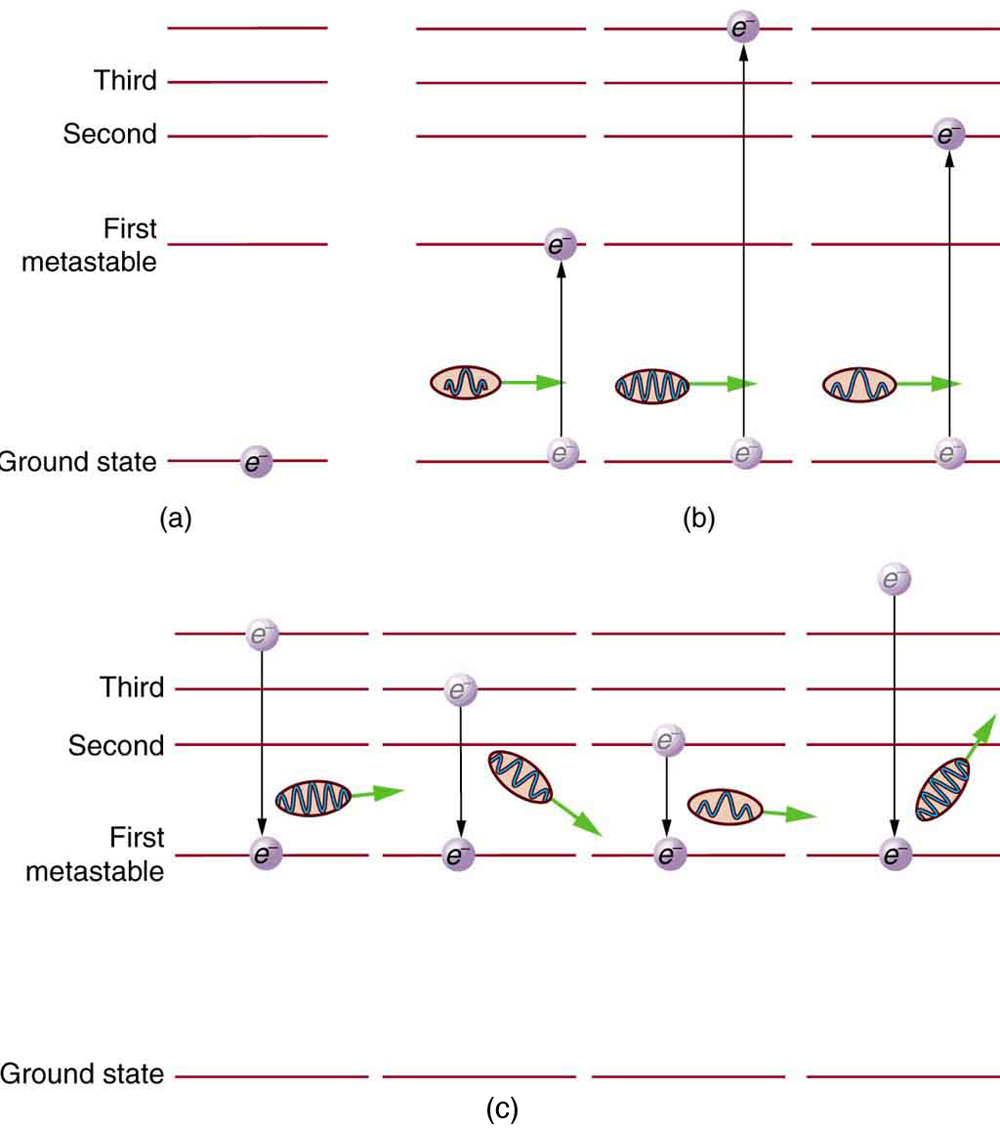
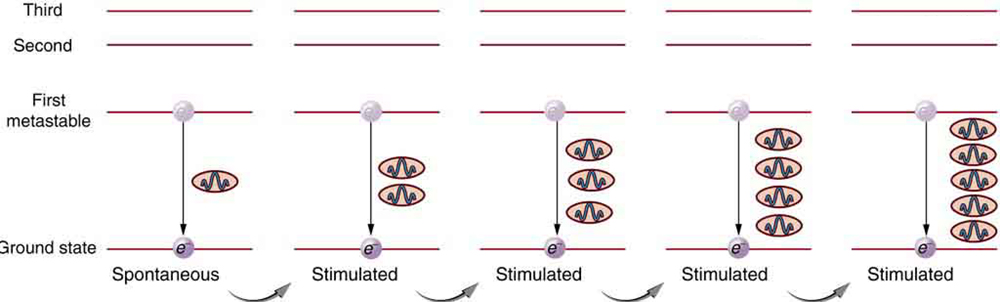
Once a population inversion is achieved, a very interesting thing can happen, as shown in [link] . An electron spontaneously falls from the metastable state, emitting a photon. This photon finds another atom in the metastable state and stimulates it to decay, emitting a second photon of the same wavelength and in phase with the first, and so on. Stimulated emission is the emission of electromagnetic radiation in the form of photons of a given frequency, triggered by photons of the same frequency. For example, an excited atom, with an electron in an energy orbit higher than normal, releases a photon of a specific frequency when the electron drops back to a lower energy orbit. If this photon then strikes another electron in the same high-energy orbit in another atom, another photon of the same frequency is released. The emitted photons and the triggering photons are always in phase, have the same polarization, and travel in the same direction. The probability of absorption of a photon is the same as the probability of stimulated emission, and so a majority of atoms must be in the metastable state to produce energy. Einstein (again Einstein, and back in 1917!) was one of the important contributors to the understanding of stimulated emission of radiation. Among other things, Einstein was the first to realize that stimulated emission and absorption are equally probable. The laser acts as a temporary energy storage device that subsequently produces a massive energy output of single-wavelength, in-phase photons.
The name laser is an acronym for light amplification by stimulated emission of radiation, the process just described. The process was proposed and developed following the advances in quantum physics. A joint Nobel Prize was awarded in 1964 to American Charles Townes (1915–), and Nikolay Basov (1922–2001) and Aleksandr Prokhorov (1916–2002), from the Soviet Union, for the development of lasers. The Nobel Prize in 1981 went to Arthur Schawlow (1921-1999) for pioneering laser applications. The original devices were called masers, because they produced microwaves. The first working laser was created in 1960 at Hughes Research labs (CA) by T. Maiman. It used a pulsed high-powered flash lamp and a ruby rod to produce red light. Today the name laser is used for all such devices developed to produce a variety of wavelengths, including microwave, infrared, visible, and ultraviolet radiation. [link] shows how a laser can be constructed to enhance the stimulated emission of radiation. Energy input can be from a flash tube, electrical discharge, or other sources, in a process sometimes called optical pumping. A large percentage of the original pumping energy is dissipated in other forms, but a population inversion must be achieved. Mirrors can be used to enhance stimulated emission by multiple passes of the radiation back and forth through the lasing material. One of the mirrors is semitransparent to allow some of the light to pass through. The laser output from a laser is a mere 1% of the light passing back and forth in a laser.

Notification Switch
Would you like to follow the 'Basic physics for medical imaging' conversation and receive update notifications?