| << Chapter < Page | Chapter >> Page > |
In the acids in the other two categories, the hydrogen atom which ionizes is attached directly to an oxygen atom.Thus, to understand acidity in these molecules, we must examine what the oxygen atom is in turn bonded to. It is very interestingto note that, in examining compounds like R-O-H, where R is an atom or group of atoms, we can get either acidic or basic properties.For examples, is a strong base, whereas is a weak acid. This means that, when ionizes in solution, the Na-O linkage ionizes, whereas when ionizes in solution, the H-O bond ionizes.
To understand this behavior, we compare the strength of the simple oxyacids , , and . The 's for these acids are found experimentally to be, respectively, 10.6,8.6, and 7.5. The acid strength for increases as we move up the periodic table in the halogen group. This means that the H-O bond ionizes more readily when the oxygenatom is bonded to a more electronegative atom.
We can add to this observation by comparing
the strengths of the acids
,
,
,
and
.
(Note that the molecular formulae are more commonly written as
,
,
,
and
.
We have written them instead to emphasize the molecular structure.)The
's
of these acids are, respectively, 7.5, 2.0, -2.7, and -8.0.In each case, the molecule with more oxygen atoms on the central Cl
atom is the stronger acid:
is more acidic than
,
Why would electronegativity play a role in acid strength? There are two conclusions we might draw. First, agreater electronegativity of the atom or atoms attached to the H-O in the oxyacid apparently results in a weaker H-O bond, which isthus more readily ionized. We know that an electronegative atom polarizes bonds by drawing the electrons in the molecule towardsit. In this case, the Cl in and the Br in must polarize the H-O bond, weakening it and facilitating the ionization of the hydrogen. In comparing to , the added oxygen atom must increase the polarization of the H-Obond, thus weakening the bond further and increasing the extent of ionization.
A second conclusion has to do with the ion created by the acid ionization. The negative ion produced has asurplus electron, and the relative energy of this ion will depend on how readily that extra electron is attracted to the atoms ofion. The more electronegative those atoms are, the stronger is the attraction. Therefore, the ion can more readily accommodate the negative charge than can the ion. And the ion can more readily accommodate the negative charge than can the ion.
We conclude that the presence of strongly electronegative atoms in an acid increases the polarization of theH-O bond, thus facilitating ionization of the acid, and increases the attraction of the extra electron to the negative ion, thusstabilizing the negative ion. Both of these factors increase the acid strength. Chemists commonly use both of these conclusions inunderstanding and predicting relative acid strength.
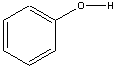
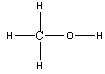
The relative acidity of carbon compounds is a major subject of organic chemistry, which we can only visit brieflyhere. In each of the carboxylic acids, the H-O group is attached to a carbonyl C=O group, which is in turn bonded to other atoms. Thecomparison we observe here is between carboxylic acid molecules, denoted as , and other organic molecules containing the H-O group, such asalcohols denoted as . (R is simply an atom or group of atoms attached to the functionalgroup.) The former are obviously acids whereas the latter group contains molecules which are generally extremely weak acids. Oneinteresting comparison is for the acid and alcohol when R is the benzene ring, . Benzoic acid, , has , whereas phenol, , has . Thus, the presence of the doubly bonded oxygen atom on the carbonatom adjacent to the O-H clearly increases the acidity of the molecule, and thus increases ionization of the O-H bond.
This observation is quite reasonable in the context of our previous conclusion. Adding an electronegativeoxygen atom in near proximity to the O-H bond both increases the polarization of the O-H bond and stabilizes the negative ionproduced by the acid ionization. In addition to the electronegativity effect, carboxylate anions, , exhibit resonance stabilization, as seen in [link] .

The resonance results in a sharing of the negative charge over several atoms, thus stabilizing the negativeion. This is a major contributing factor in the acidity of carboxylic acids versus alcohols.
Strong acids have a higher percent ionization than do weak acids. Why don't we use percent ionization as ameasure of acid strength, rather than ?
Using the data in [link] for nitrous acid, plot versus , the initial concentration of the acid, and versus the equilibrium concentration of the acid. On a second graph, plot versus , the initial concentration of the acid, and versus the equilibrium concentration of the acid. Which of these results gives a straight line? Using the equilibrium constant expression,explain your answer.
Using Le Châtelier's principle, explain why the concentration of is much lower in acidic solution than it is in neutral solution.
We considered mixing a strong base with a weak acid, but we did not consider mixing a strong acid with a weakacid. Consider mixing 0.1M and 0.1M . Predict the pH of the solution and the percent ionization of thenitrous acid. Rationalize your prediction using Le Châtelier's principle.
Imagine taking a 0.5M solution of nitrous acid and slowing adding water to it. Looking at [link] , we see that, as the concentration of nitrous acid decreases, the percent ionization increases. Bycontrast, decreases. Rationalize these results using Le Châtelier's principle.
We observed that mixing a strong acid and a strong base, in equal amounts and concentrations, produces aneutral solution, and that mixing a strong base with a weak acid, in equal amounts and concentrations, produces a basic solution.Imagine mixing a weak acid and a weak base, in equal amounts and concentrations. Predict whether the resulting solution will beacidic, basic, or neutral, and explain your prediction.
Using the electronegativity arguments presented above , explain why, in general, compounds like M-O-H are bases rather thanacids, when M is a metal atom. Predict the relationship between the properties of the metal atom M and the strength of the base .
Ionization of sulfuric acid produces , which is also an acid. However, is a much weaker acid than . Using the conclusions from above , explain why is a much weaker acid.
Predict and explain the relative acid strengths of and . Predict and explain the relative acid strengths of and .
Using arguments from above , predict and explain the relative acidity of phenol and methanol .

Notification Switch
Would you like to follow the 'General chemistry ii' conversation and receive update notifications?