| << Chapter < Page | Chapter >> Page > |
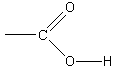
Unlike our previous salt solution, a measurement in this case reveals that the pH of the product salt solution is 9.4, so the solution is basic. Thus,mixing equal molar quantities of strong base with weak acid produces a basic solution. In essence, the weak acid does not fullyneutralize the strong base. To understand this, we examine the behavior of sodium acetate in solution. Since the pH is greaterthan 7, then there is an excess of ions in solution relative to pure water. These ions must have come from the reaction of sodium acetate with the water. Therefore, thenegative acetate ions in solution must behave as a base, accepting positive hydrogen ions:
The reaction of an ion with water to form either an acid or a base solution is referred to as hydrolysis . From this example, the salt of a weak acid behaves as a base in water, resulting in a pH greater than7.
To understand the extent to which the hydrolysis of the negative ion occurs, we need to know theequilibrium constant for this reaction. This turns out to be determined by the acid ionization constant for . To see this, we write the equilibrium constant for the hydrolysisof as
Multiplying numerator and denominator by , we find that
Therefore, for the hydrolysis of acetate ions in solution, . This is fairly small, so the acetate ion is a very weakbase.
We now have a fairly complete quantitative description of acid-base equilibrium. To complete our understandingof acid-base equilibrium, we need a predictive model which relates acid strength or base strength to molecular properties. In general,we expect that the strength of an acid is related either to the relative ease by which it can donate a hydrogen ion or by therelative stability of the remaining negative ion formed after the departure of the hydrogen ion.
To begin, we note that there are three basic categories of acids which we have examined in this study. First,there are simple binary acids :
We consider first the simple binary acids. , , and are all strong acids, whereas is a weak acid. In comparing the experimental values of values in [link] , we note that the acid strength increases in the order . This means that the hydrogen ion can more readily separate from thecovalent bond with the halogen atom (X) as we move down the periodic table. This is reasonable, because the strength of the H-Xbond also decreases as we move down the periodic table, as shown in [link] .
| Bond Energy ( ) | ||
|---|---|---|
| 3.1 | 567.7 | |
| -6.0 | 431.6 | |
| -9.0 | 365.9 | |
| -9.5 | 298.0 |
The decreasing strength of the H-X bond is primarily due to the increase is the size of the X atom as we movedown the periodic table. We conclude that one factor which influences acidity is the strength of the H-X bond: a weaker bondproduces a stronger acid, and vice versa.

Notification Switch
Would you like to follow the 'General chemistry ii' conversation and receive update notifications?