| << Chapter < Page | Chapter >> Page > |
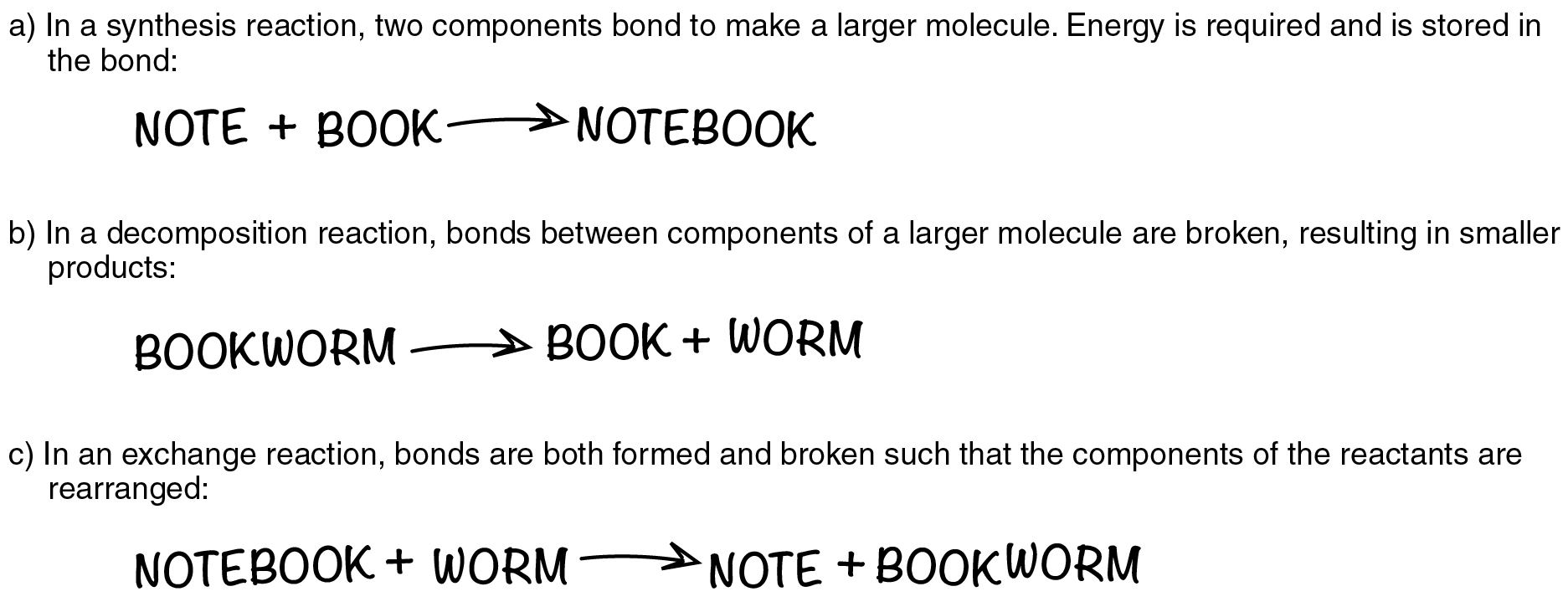
In the second example, ammonia is catabolized into its smaller components, and the potential energy that had been stored in its bonds is released. Such reactions are referred to as decomposition reactions. A decomposition reaction is a chemical reaction that breaks down or “de-composes” something larger into its constituent parts (see [link] b ). The general equation for a decomposition reaction is: .
An exchange reaction is a chemical reaction in which both synthesis and decomposition occur, chemical bonds are both formed and broken, and chemical energy is absorbed, stored, and released (see [link] c ). The simplest form of an exchange reaction might be: . Notice that, to produce these products, B and C had to break apart in a decomposition reaction, whereas A and B had to bond in a synthesis reaction. A more complex exchange reaction might be: . Another example might be: .
In theory, any chemical reaction can proceed in either direction under the right conditions. Reactants may synthesize into a product that is later decomposed. Reversibility is also a quality of exchange reactions. For instance, could then reverse to . This reversibility of a chemical reaction is indicated with a double arrow: . Still, in the human body, many chemical reactions do proceed in a predictable direction, either one way or the other. You can think of this more predictable path as the path of least resistance because, typically, the alternate direction requires more energy.
If you pour vinegar into baking soda, the reaction is instantaneous; the concoction will bubble and fizz. But many chemical reactions take time. A variety of factors influence the rate of chemical reactions. This section, however, will consider only the most important in human functioning.
If chemical reactions are to occur quickly, the atoms in the reactants have to have easy access to one another. Thus, the greater the surface area of the reactants, the more readily they will interact. When you pop a cube of cheese into your mouth, you chew it before you swallow it. Among other things, chewing increases the surface area of the food so that digestive chemicals can more easily get at it. As a general rule, gases tend to react faster than liquids or solids, again because it takes energy to separate particles of a substance, and gases by definition already have space between their particles. Similarly, the larger the molecule, the greater the number of total bonds, so reactions involving smaller molecules, with fewer total bonds, would be expected to proceed faster.
In addition, recall that some elements are more reactive than others. Reactions that involve highly reactive elements like hydrogen proceed more quickly than reactions that involve less reactive elements. Reactions involving stable elements like helium are not likely to happen at all.

Notification Switch
Would you like to follow the 'Ucd bis2a intro to biology v1.2' conversation and receive update notifications?