| << Chapter < Page | Chapter >> Page > |
Nearly all chemical reactions occur at a faster rate at higher temperatures. Recall that kinetic energy is the energy of matter in motion. The kinetic energy of subatomic particles increases in response to increases in thermal energy. The higher the temperature, the faster the particles move, and the more likely they are to come in contact and react.
If just a few people are dancing at a club, they are unlikely to step on each other’s toes. But as more and more people get up to dance—especially if the music is fast—collisions are likely to occur. It is the same with chemical reactions: the more particles present within a given space, the more likely those particles are to bump into one another. This means that chemists can speed up chemical reactions not only by increasing the concentration of particles—the number of particles in the space—but also by decreasing the volume of the space, which would correspondingly increase the pressure. If there were 100 dancers in that club, and the manager abruptly moved the party to a room half the size, the concentration of the dancers would double in the new space, and the likelihood of collisions would increase accordingly.
For two chemicals in nature to react with each other they first have to come into contact, and this occurs through random collisions. Because heat helps increase the kinetic energy of atoms, ions, and molecules, it promotes their collision. But in the body, extremely high heat—such as a very high fever—can damage body cells and be life-threatening. On the other hand, normal body temperature is not high enough to promote the chemical reactions that sustain life. That is where catalysts come in.
In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any change. You can think of a catalyst as a chemical change agent. They help increase the rate and force at which atoms, ions, and molecules collide, thereby increasing the probability that their valence shell electrons will interact.
The most important catalysts in the human body are enzymes. An enzyme is a catalyst composed of protein or ribonucleic acid (RNA), both of which will be discussed later in this chapter. Like all catalysts, enzymes work by lowering the level of energy that needs to be invested in a chemical reaction. A chemical reaction’s activation energy is the “threshold” level of energy needed to break the bonds in the reactants. Once those bonds are broken, new arrangements can form. Without an enzyme to act as a catalyst, a much larger investment of energy is needed to ignite a chemical reaction ( [link] ).
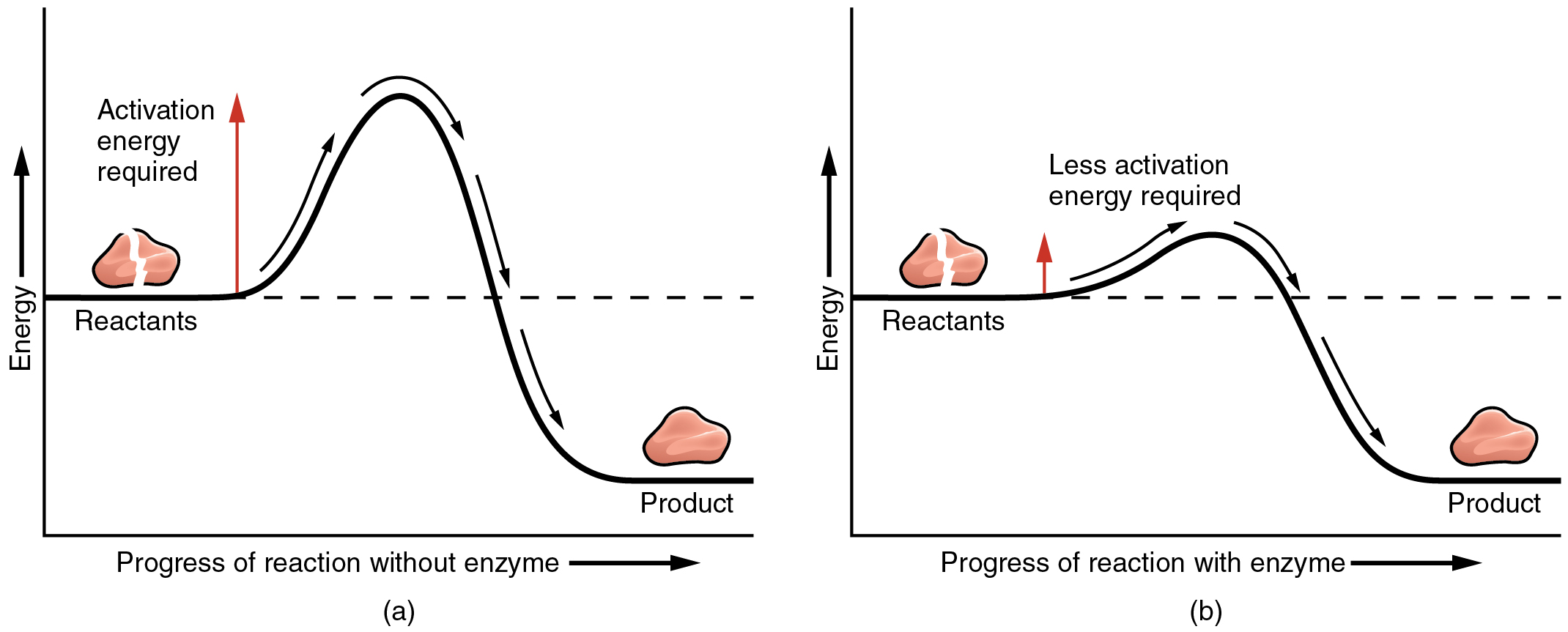
Enzymes are critical to the body’s healthy functioning. They assist, for example, with the breakdown of food and its conversion to energy. In fact, most of the chemical reactions in the body are facilitated by enzymes.
Chemical reactions, in which chemical bonds are broken and formed, require an initial investment of energy. Kinetic energy, the energy of matter in motion, fuels the collisions of atoms, ions, and molecules that are necessary if their old bonds are to break and new ones to form. All molecules store potential energy, which is released when their bonds are broken.
Four forms of energy essential to human functioning are: chemical energy, which is stored and released as chemical bonds are formed and broken; mechanical energy, which directly powers physical activity; radiant energy, emitted as waves such as in sunlight; and electrical energy, the power of moving electrons.
Chemical reactions begin with reactants and end with products. Synthesis reactions bond reactants together, a process that requires energy, whereas decomposition reactions break the bonds within a reactant and thereby release energy. In exchange reactions, bonds are both broken and formed, and energy is exchanged.
The rate at which chemical reactions occur is influenced by several properties of the reactants: temperature, concentration and pressure, and the presence or absence of a catalyst. An enzyme is a catalytic protein that speeds up chemical reactions in the human body.

Notification Switch
Would you like to follow the 'Biological reactions and catalysts' conversation and receive update notifications?