| << Chapter < Page | Chapter >> Page > |
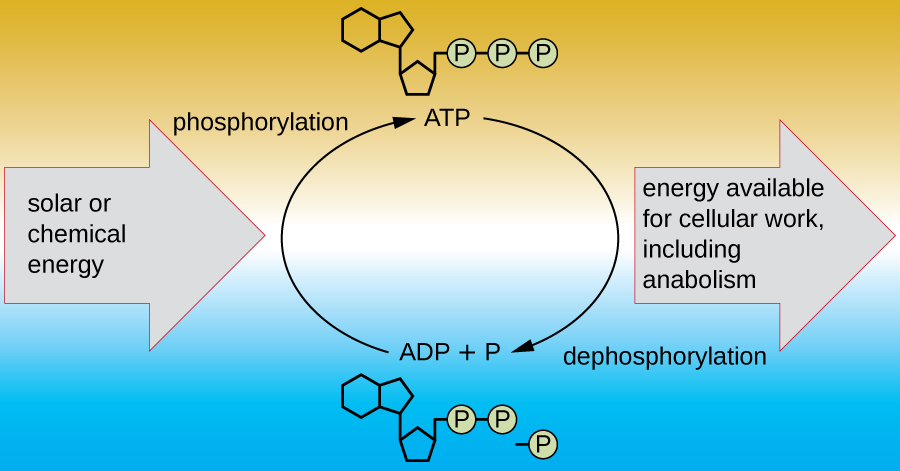
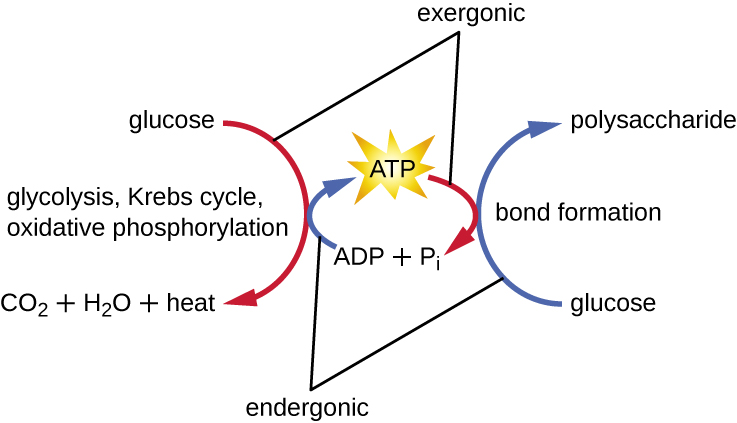
A living cell must be able to handle the energy released during catabolism in a way that enables the cell to store energy safely and release it for use only as needed. Living cells accomplish this by using the compound adenosine triphosphate (ATP) . ATP is often called the “energy currency” of the cell, and, like currency, this versatile compound can be used to fill any energy need of the cell. At the heart of ATP is a molecule of adenosine monophosphate (AMP) , which is composed of an adenine molecule bonded to a ribose molecule and a single phosphate group. Ribose is a five-carbon sugar found in RNA, and AMP is one of the nucleotides in RNA. The addition of a second phosphate group to this core molecule results in the formation of adenosine diphosphate (ADP) ; the addition of a third phosphate group forms ATP ( [link] ). Adding a phosphate group to a molecule, a process called phosphorylation , requires energy. Phosphate groups are negatively charged and thus repel one another when they are arranged in series, as they are in ADP and ATP. This repulsion makes the ADP and ATP molecules inherently unstable. Thus, the bonds between phosphate groups (one in ADP and two in ATP) are called high-energy phosphate bond s . When these high-energy bonds are broken to release one phosphate (called inorganic phosphate [P i ] ) or two connected phosphate groups (called pyrophosphate [PP i ] ) from ATP through a process called dephosphorylation , energy is released to drive endergonic reaction s ( [link] ).
A substance that helps speed up a chemical reaction is a catalyst . Catalysts are not used or changed during chemical reactions and, therefore, are reusable. Whereas inorganic molecules may serve as catalysts for a wide range of chemical reactions, proteins called enzyme s serve as catalysts for biochemical reactions inside cells. Enzymes thus play an important role in controlling cellular metabolism.
An enzyme functions by lowering the activation energy of a chemical reaction inside the cell. Activation energy is the energy needed to form or break chemical bonds and convert reactants to products ( [link] ). Enzymes lower the activation energy by binding to the reactant molecules and holding them in such a way as to speed up the reaction.
The chemical reactants to which an enzyme binds are called substrate s , and the location within the enzyme where the substrate binds is called the enzyme’s active site . The characteristics of the amino acids near the active site create a very specific chemical environment within the active site that induces suitability to binding, albeit briefly, to a specific substrate (or substrates). Due to this jigsaw puzzle-like match between an enzyme and its substrates, enzyme s are known for their specificity. In fact, as an enzyme binds to its substrate(s), the enzyme structure changes slightly to find the best fit between the transition state (a structural intermediate between the substrate and product) and the active site, just as a rubber glove molds to a hand inserted into it. This active-site modification in the presence of substrate, along with the simultaneous formation of the transition state, is called induced fit ( [link] ). Overall, there is a specifically matched enzyme for each substrate and, thus, for each chemical reaction; however, there is some flexibility as well. Some enzymes have the ability to act on several different structurally related substrates.

Notification Switch
Would you like to follow the 'Microbiology' conversation and receive update notifications?