| << Chapter < Page | Chapter >> Page > |
What you should notice is that ΔG and ΔE have different signage: When ΔG is positive, ΔE is negative and when ΔG is negative ΔE is positive. For a review see Red/Ox discussion in the Bis2A Discussion Manual.
As you may have figured out, all kinds of compounds can be used in red/ox reactions in the cell. Making sense of all of this information and ranking potential red/ox pairs can be confusing. A tool has been developed to rate red/ox half reactions based on their E 0 ' values. Whether a particular compound can act as an electron donor (reductant) or electron acceptor (oxidant) sill depend on what other compound it is interacting with. The electron tower ranks a variety of common compounds (their half reactions) from most negative E 0 ' , compounds that readily get rid of electrons, to the most positive E 0 ' , compound most likely to accept electrons. The tower organizes these half reactions based on the ability of electrons to accept electrons, with the most electronegative at the bottom of the tower. So the most negative E 0 ' values are at the top. In addition each half reaction is written by convention with the oxidized form on the left/followed by the reduced form. For example the half reaction for the reduction of NAD + to NADH is written: NAD + /NADH + 2e - . An electron tower is shown in figure 2 below.
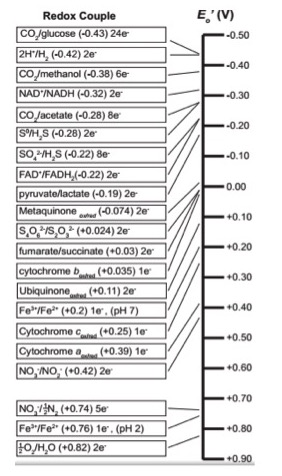
The right and left sides of the chemical reactions in the redox tower are separated by a "/". The form of the compound on the left of the slash is____________, and the form of the compound on the right of the slash is______________.
a
Remember, by convention the tower half reactions are written with the oxidized form of the compound on the left and the reduced form on the right. Compounds that make excellent electron donors, remember that first class of compounds we discussed earlier, are found at the top of the tower. Compounds such as Glucose and Hydrogen gas are excellent electron donors. Notice, that they are found on the right hand side of the red/ox pair half reactions. At the other end of the tower lies compounds that make excellent terminal electron acceptors, such as Oxygen and Nitrite, these compounds are found on the left side of the red/ox pair and have a positive E 0 ' value.
The tower is a tool to help determine whether a compound can act as an electron donor or an electron acceptor. Here lies the beauty of the that third class of compounds discussed above. These intermediate carriers, such as cytochromes or quinones, can act as either acceptor or donor depending upon their red/ox state and whether the other component in the red/ox reaction has a higher or lower E 0 ' value.
Once metaquinone has been reduced, it can now act as an electron donor to any compound that sits lower (below it) on the electron tower: any compound that has a higher E 0 ' value. Possible electron acceptors include cytochrome b ox with an E 0 ' value of 0.035 eV; or ubiquinone ox with an E 0 ' of 0.11 eV. Remember that the oxidized forms lie on the left side of the half reaction.
Which of the following could be used as an electron acceptor for Ubiquinone red
C
Red/Ox reactions involve the movement of electrons from one compound to another. As electrons move from, the energy released can be captured by the cell to do work, such as synthesize ATP. Every red/ox reaction can be thought of as 2 half reactions, in one reaction a compound looses electrons and in the second reaction a different compound gains electrons. The amount of potential energy released is the difference in each half reactions reduction potential, E 0 ' . The electron tower is a tool that ranks different common half reactions (and therefore various compounds) based on how likely they are to donate or accept electrons. The lower, more negative, the electrochemical potential for each half reaction, the higher it sits in the electron tower. Reduced compounds can donate electrons to oxidized compounds that are below it on the electron tower. Oxidized compounds can accept electrons from any compound that are above it in the electron tower. The use of the electron tower will be more evident as we discuss electron transport chains in a few modules.

Notification Switch
Would you like to follow the 'Ucd bis2a intro to biology v1.2' conversation and receive update notifications?