| << Chapter < Page | Chapter >> Page > |
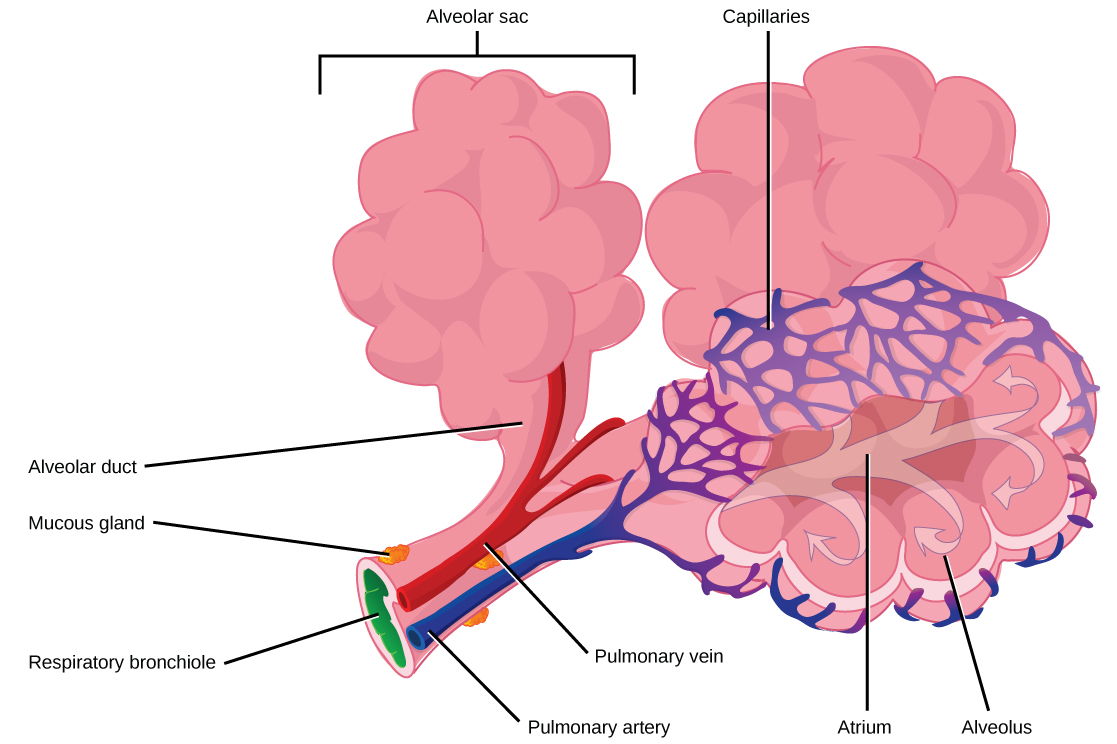
The terminal bronchioles subdivide into microscopic branches called respiratory bronchioles. The respiratory bronchioles subdivide into several alveolar ducts. Numerous alveoli and alveolar sacs surround the alveolar ducts. The alveolar sacs resemble bunches of grapes tethered to the end of the bronchioles ( [link] ). Alveoli are made of thin-walled parenchymal cells, typically one-cell thick, that look like tiny bubbles within the sacs. Alveoli are in direct contact with capillaries (one-cell thick) of the circulatory system. Such intimate contact ensures that oxygen will diffuse from alveoli into the blood and be distributed to the cells of the body. In addition, the carbon dioxide that was produced by cells as a waste product will diffuse from the blood into alveoli to be exhaled.
Once the oxygen diffuses across the alveoli, it enters the bloodstream and is transported to the tissues where it is unloaded, and carbon dioxide diffuses out of the blood and into the alveoli to be expelled from the body. Although gas exchange is a continuous process, the oxygen and carbon dioxide are transported by different mechanisms.
Although oxygen dissolves in blood, only a small amount of oxygen is transported this way. Only 1.5 percent of oxygen in the blood is dissolved directly into the blood itself. Most oxygen—98.5 percent—is bound to a protein called hemoglobin and carried to the tissues.
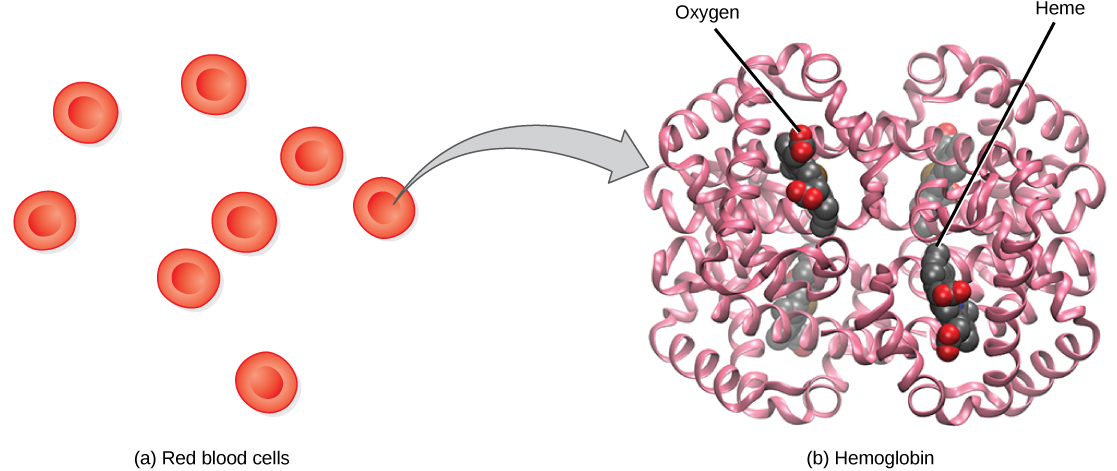
Hemoglobin , or Hb, is a protein molecule found in red blood cells (erythrocytes) made of four subunits: two alpha subunits and two beta subunits ( [link] ). Each subunit surrounds a central heme group that contains iron and binds one oxygen molecule, allowing each hemoglobin molecule to bind four oxygen molecules. Molecules with more oxygen bound to the heme groups are brighter red. As a result, oxygenated arterial blood where the Hb is carrying four oxygen molecules is bright red, while venous blood that is deoxygenated is darker red.
Carbon dioxide molecules are transported in the blood from body tissues to the lungs by one of three methods: dissolution directly into the blood, binding to hemoglobin, or carried as a bicarbonate ion. Several properties of carbon dioxide in the blood affect its transport. First, carbon dioxide is more soluble in blood than oxygen. About 5 to 7 percent of all carbon dioxide is dissolved in the plasma. Second, carbon dioxide can bind to plasma proteins or can enter red blood cells and bind to hemoglobin. This form transports about 10 percent of the carbon dioxide. When carbon dioxide binds to hemoglobin, a molecule called carbaminohemoglobin is formed. Binding of carbon dioxide to hemoglobin is reversible. Therefore, when it reaches the lungs, the carbon dioxide can freely dissociate from the hemoglobin and be expelled from the body.
Third, the majority of carbon dioxide molecules (85 percent) are carried as part of the bicarbonate buffer system. In this system, carbon dioxide diffuses into the red blood cells. Carbonic anhydrase (CA) within the red blood cells quickly converts the carbon dioxide into carbonic acid (H 2 CO 3 ). Carbonic acid is an unstable intermediate molecule that immediately dissociates into bicarbonate ions and hydrogen (H + ) ions. Since carbon dioxide is quickly converted into bicarbonate ions, this reaction allows for the continued uptake of carbon dioxide into the blood down its concentration gradient. It also results in the production of H + ions. If too much H + is produced, it can alter blood pH. However, hemoglobin binds to the free H + ions and thus limits shifts in pH. The newly synthesized bicarbonate ion is transported out of the red blood cell into the plasma of the blood in exchange for a chloride ion (Cl - ); this is called the chloride shift. When the blood reaches the lungs, the bicarbonate ion is transported back into the red blood cell in exchange for the chloride ion. The H + ion dissociates from the hemoglobin and binds to the bicarbonate ion. This produces the carbonic acid intermediate, which is converted back into carbon dioxide through the enzymatic action of CA. The carbon dioxide produced is expelled through the lungs during exhalation.
The benefit of the bicarbonate buffer system is that carbon dioxide is “soaked up” into the blood with little change to the pH of the system. This is important because it takes only a small change in the overall pH of the body for severe injury or death to result. The presence of this bicarbonate buffer system also allows for people to travel and live at high altitudes: When the partial pressure of oxygen and carbon dioxide change at high altitudes, the bicarbonate buffer system adjusts to regulate carbon dioxide while maintaining the correct pH in the body.

Notification Switch
Would you like to follow the 'Principles of biology' conversation and receive update notifications?