| << Chapter < Page | Chapter >> Page > |
According to geologists, if there were no heat source, Earth should have cooled to its present temperature in no more than 1 billion years. Yet, Earth is more than 4 billion years old. Why is Earth cooling so slowly? The answer is nuclear radioactivity, that is, high-energy particles produced in radioactive decays heat Earth from the inside ( [link] ).
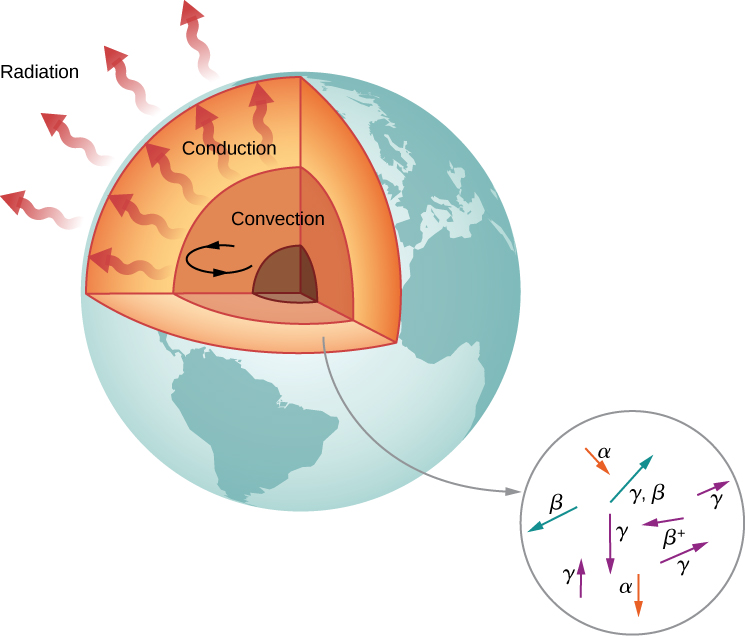
Candidate nuclei for this heating model are , which possess half-lives similar to or longer than the age of Earth. The energy produced by these decays (per second per cubic meter) is small, but the energy cannot escape easily, so Earth’s core is very hot. Thermal energy in Earth’s core is transferred to Earth’s surface and away from it through the processes of convection, conduction, and radiation.
What is the key difference and the key similarity between beta ( ) decay and alpha decay?
What is the difference between rays and characteristic X-rays and visible light?
Gamma ( γ ) rays are produced by nuclear interactions and X-rays and light are produced by atomic interactions. Gamma rays are typically shorter wavelength than X-rays, and X-rays are shorter wavelength than light.
What characteristics of radioactivity show it to be nuclear in origin and not atomic?
Consider [link] . If the magnetic field is replaced by an electric field pointed in toward the page, in which directions will the -, -, and rays bend?
Assume a rectangular coordinate system with an xy -plane that corresponds to the plane of the paper. bends into the page (trajectory parabolic in the xz -plane); bends into the page (trajectory parabolic in the xz -plane); and is unbent.
Why is Earth’s core molten?
undergoes alpha decay. (a) Write the reaction equation. (b) Find the energy released in the decay.
(a) Calculate the energy released in the decay of . (b) What fraction of the mass of a single is destroyed in the decay? The mass of is 234.043593 u. (c) Although the fractional mass loss is large for a single nucleus, it is difficult to observe for an entire macroscopic sample of uranium. Why is this?
a. 4.273 MeV; b. ; c. Since is a slowly decaying substance, only a very small number of nuclei decay on human timescales; therefore, although those nuclei that decay lose a noticeable fraction of their mass, the change in the total mass of the sample is not detectable for a macroscopic sample.
The particles emitted in the decay of (tritium) interact with matter to create light in a glow-in-the-dark exit sign. At the time of manufacture, such a sign contains 15.0 Ci of . (a) What is the mass of the tritium? (b) What is its activity 5.00 y after manufacture?
(a) Write the complete decay equation for a major waste product of nuclear reactors. (b) Find the energy released in the decay.
a. ; b. 0.546 MeV
Write a nuclear decay reaction that produces the nucleus. ( Hint: The parent nuclide is a major waste product of reactors and has chemistry similar to calcium, so that it is concentrated in bones if ingested.)
Write the complete decay equation in the complete notation for the beta ( ) decay of (tritium), a manufactured isotope of hydrogen used in some digital watch displays, and manufactured primarily for use in hydrogen bombs.
If a 1.50-cm-thick piece of lead can absorb of the rays from a radioactive source, how many centimeters of lead are needed to absorb all but of the rays?
An electron can interact with a nucleus through the beta-decay process:
.
(a) Write the complete reaction equation for electron capture by .
(b) Calculate the energy released.
a. ; b. 0.862 MeV
(a) Write the complete reaction equation for electron capture by
(b) Calculate the energy released.
A rare decay mode has been observed in which emits a nucleus. (a) The decay equation is . Identify the nuclide . (b) Find the energy emitted in the decay. The mass of is 222.015353 u.
a. ; b. 33.05 MeV

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?