| << Chapter < Page | Chapter >> Page > |
In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (BEN) , which is defined by
This quantity is the average energy required to remove an individual nucleon from a nucleus—analogous to the ionization energy of an electron in an atom. If the BEN is relatively large, the nucleus is relatively stable. BEN values are estimated from nuclear scattering experiments.
A graph of binding energy per nucleon versus atomic number A is given in [link] . This graph is considered by many physicists to be one of the most important graphs in physics. Two notes are in order. First, typical BEN values range from 6–10 MeV, with an average value of about 8 MeV. In other words, it takes several million electron volts to pry a nucleon from a typical nucleus, as compared to just 13.6 eV to ionize an electron in the ground state of hydrogen. This is why nuclear force is referred to as the “strong” nuclear force.
Second, the graph rises at low A , peaks very near iron and then tapers off at high A . The peak value suggests that the iron nucleus is the most stable nucleus in nature (it is also why nuclear fusion in the cores of stars ends with Fe). The reason the graph rises and tapers off has to do with competing forces in the nucleus. At low values of A , attractive nuclear forces between nucleons dominate over repulsive electrostatic forces between protons. But at high values of A , repulsive electrostatic forces between forces begin to dominate, and these forces tend to break apart the nucleus rather than hold it together.
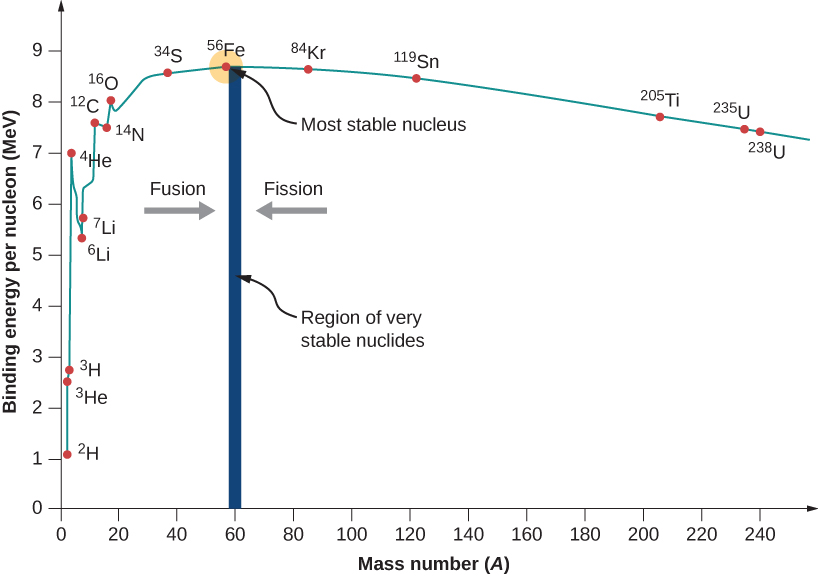
As we will see, the BEN-versus- A graph implies that nuclei divided or combined release an enormous amount of energy. This is the basis for a wide range of phenomena, from the production of electricity at a nuclear power plant to sunlight.
These masses are and Thus we have,
Noting that we find
Since the total binding energy per nucleon is
Check Your Understanding If the binding energy per nucleon is large, does this make it harder or easier to strip off a nucleon from a nucleus?
harder
Explain why a bound system should have less mass than its components. Why is this not observed traditionally, say, for a building made of bricks?
A bound system should have less mass than its components because of energy-mass equivalence If the energy of a system is reduced, the total mass of the system is reduced. If two bricks are placed next to one another, the attraction between them is purely gravitational, assuming the bricks are electrically neutral. The gravitational force between the bricks is relatively small (compared to the strong nuclear force), so the mass defect is much too small to be observed. If the bricks are glued together with cement, the mass defect is likewise small because the electrical interactions between the electrons involved in the bonding are still relatively small.
Why is the number of neutrons greater than the number of protons in stable nuclei that have an A greater than about 40? Why is this effect more pronounced for the heaviest nuclei?
To obtain the most precise value of the binding energy per nucleon, it is important to take into account forces between nucleons at the surface of the nucleus. Will surface effects increase or decrease estimates of BEN?
Nucleons at the surface of a nucleus interact with fewer nucleons. This reduces the binding energy per nucleon, which is based on an average over all the nucleons in the nucleus.
How much energy would be released if six hydrogen atoms and six neutrons were combined to form
92.4 MeV
Find the mass defect and the binding energy for the helium-4 nucleus.
is among the most tightly bound of all nuclides. It makes up more than of natural iron. Note that has even numbers of protons and neutrons. Calculate the binding energy per nucleon for and compare it with the approximate value obtained from the graph in [link] .
is the heaviest stable nuclide, and its BEN is low compared with medium-mass nuclides. Calculate BEN for this nucleus and compare it with the approximate value obtained from the graph in [link] .
(a) Calculate BEN for , the rarer of the two most common uranium isotopes; (b) Calculate BEN for . (Most of uranium is .)
a. 7.570 MeV; b.
The fact that BEN peaks at roughly implies that the range of the strong nuclear force is about the diameter of this nucleus.
(a) Calculate the diameter of nucleus.
(b) Compare BEN for . The first is one of the most tightly bound nuclides, whereas the second is larger and less tightly bound.

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?