| << Chapter < Page | Chapter >> Page > |
Consider the special case of a free particle. A free particle experiences no force Based on [link] , this requires only that
For simplicity, we set . Schrӧdinger’s equation then reduces to
A valid solution to this equation is
Not surprisingly, this solution contains an imaginary number because the differential equation itself contains an imaginary number. As stressed before, however, quantum-mechanical predictions depend only on , which yields completely real values. Notice that the real plane-wave solutions, and do not obey Schrödinger’s equation. The temptation to think that a wave function can be seen, touched, and felt in nature is eliminated by the appearance of an imaginary number. In Schrӧdinger’s theory of quantum mechanics, the wave function is merely a tool for calculating things.
If the potential energy function ( U ) does not depend on time, it is possible to show that
satisfies Schrӧdinger’s time-dependent equation, where is a time -independent function and is a space -independent function. In other words, the wave function is separable into two parts: a space-only part and a time-only part. The factor is sometimes referred to as a time-modulation factor since it modifies the space-only function. According to de Broglie, the energy of a matter wave is given by , where E is its total energy. Thus, the above equation can also be written as
Any linear combination of such states (mixed state of energy or momentum) is also valid solution to this equation. Such states can, for example, describe a localized particle (see [link] )
Check Your Understanding A particle with mass m is moving along the x -axis in a potential given by the potential energy function . Compute the product Express your answer in terms of the time-independent wave function,
Combining [link] and [link] , Schrödinger’s time-dependent equation reduces to
where E is the total energy of the particle (a real number). This equation is called Schrӧdinger’s time-independent equation . Notice that we use “big psi” for the time-dependent wave function and “little psi” for the time-independent wave function. The wave-function solution to this equation must be multiplied by the time-modulation factor to obtain the time-dependent wave function.
In the next sections, we solve Schrӧdinger’s time-independent equation for three cases: a quantum particle in a box, a simple harmonic oscillator, and a quantum barrier. These cases provide important lessons that can be used to solve more complicated systems. The time-independent wave function solutions must satisfy three conditions:
The first condition avoids sudden jumps or gaps in the wave function. The second condition requires the wave function to be smooth at all points, except in special cases. (In a more advanced course on quantum mechanics, for example, potential spikes of infinite depth and height are used to model solids). The third condition requires the wave function be normalizable. This third condition follows from Born’s interpretation of quantum mechanics. It ensures that is a finite number so we can use it to calculate probabilities.
Check Your Understanding Which of the following wave functions is a valid wave-function solution for Schrӧdinger’s equation?
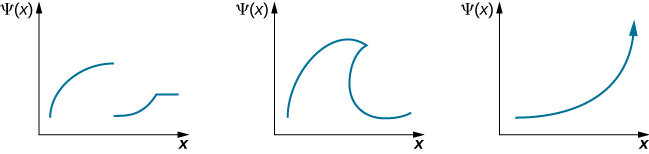
None. The first function has a discontinuity; the second function is double-valued; and the third function diverges so is not normalizable.
What is the difference between a wave function and a wave function for the same particle?
If a quantum particle is in a stationary state, does it mean that it does not move?
No, it means that predictions about the particle (expressed in terms of probabilities) are time-independent.
Explain the difference between time-dependent and -independent Schrӧdinger’s equations.
Suppose a wave function is discontinuous at some point. Can this function represent a quantum state of some physical particle? Why? Why not?
No, because the probability of the particle existing in a narrow (infinitesimally small) interval at the discontinuity is undefined.
Carrying out the derivatives yields
Show that is a valid solution to Schrӧdinger’s time-dependent equation.
Show that and do not obey Schrӧdinger’s time-dependent equation.
Carrying out the derivatives (as above) for the sine function gives a cosine on the right side the equation, so the equality fails. The same occurs for the cosine solution.
Show that when and are solutions to the time-dependent Schrӧdinger equation and A , B are numbers, then a function that is a superposition of these functions is also a solution: .
A particle with mass m is described by the following wave function: , where A , B , and k are constants. Assuming that the particle is free, show that this function is the solution of the stationary Schrӧdinger equation for this particle and find the energy that the particle has in this state.
Find the expectation value of the kinetic energy for the particle in the state, . What conclusion can you draw from your solution?
Find the expectation value of the square of the momentum squared for the particle in the state, . What conclusion can you draw from your solution?
; The particle has definite momentum and therefore definite momentum squared.
A free proton has a wave function given by .
The coefficient of x is inverse meters and the coefficient on t is inverse seconds Find its momentum and energy.

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?