| << Chapter < Page | Chapter >> Page > |
The free electron model explains many important properties of conductors but is weak in at least two areas. First, it assumes a constant potential energy within the solid. (Recall that a constant potential energy is associated with no forces.) [link] compares the assumption of a constant potential energy (dotted line) with the periodic Coulomb potential, which drops as at each lattice point, where r is the distance from the ion core (solid line). Second, the free electron model assumes an impenetrable barrier at the surface. This assumption is not valid, because under certain conditions, electrons can escape the surface—such as in the photoelectric effect. In addition to these assumptions, the free electron model does not explain the dramatic differences in electronic properties of conductors, semiconductors, and insulators. Therefore, a more complete model is needed.
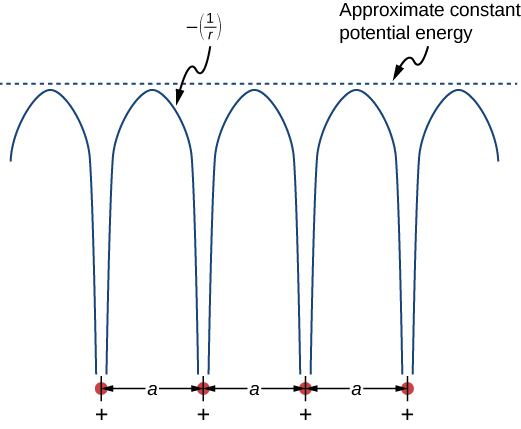
We can produce an improved model by solving Schrödinger’s equation for the periodic potential shown in [link] . However, the solution requires technical mathematics far beyond our scope. We again seek a qualitative argument based on quantum mechanics to find a way forward.
We first review the argument used to explain the energy structure of a covalent bond. Consider two identical hydrogen atoms so far apart that there is no interaction whatsoever between them. Further suppose that the electron in each atom is in the same ground state: a 1 s electron with an energy of (ignore spin). When the hydrogen atoms are brought closer together, the individual wave functions of the electrons overlap and, by the exclusion principle, can no longer be in the same quantum state, which splits the original equivalent energy levels into two different energy levels. The energies of these levels depend on the interatomic distance, ( [link] ).
If four hydrogen atoms are brought together, four levels are formed from the four possible symmetries—a single sine wave “hump” in each well, alternating up and down, and so on. In the limit of a very large number N of atoms, we expect a spread of nearly continuous bands of electronic energy levels in a solid (see [link] (c)). Each of these bands is known as an energy band . (The allowed states of energy and wave number are still technically quantized, but for large numbers of atoms, these states are so close together that they are consider to be continuous or “in the continuum.”)
Energy bands differ in the number of electrons they hold. In the 1 s and 2 s energy bands, each energy level holds up to two electrons (spin up and spin down), so this band has a maximum occupancy of 2 N electrons. In the 2 p energy band, each energy level holds up to six electrons, so this band has a maximum occupancy of 6 N electrons ( [link] ).

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?