| << Chapter < Page | Chapter >> Page > |
As we discuss later, the total wave function of two electrons must be antisymmetric on exchange. For example, two electrons bound to a hydrogen molecule can be in a space-symmetric state with antiparallel spins or space-antisymmetric state with parallel spins . The state with antiparallel spins is energetically favorable and therefore used in covalent bonding. If the protons are drawn too closely together, however, repulsion between the protons becomes important. (In other molecules, this effect is supplied by the exclusion principle.) As a result, reaches an equilibrium separation of about 0.074 nm with a binding energy is 4.52 eV.
Visit this PBS Learning Media tutorial and interactive simulation to explore the attractive and repulsive forces that act on atomic particles and covalent bonding in a molecule.
Quantum mechanics excludes many types of molecules. For example, the molecule does not form, because if a third H atom approaches diatomic hydrogen, the wave function of the electron in this atom overlaps the electrons in the other two atoms. If all three electrons are in the ground states of their respective atoms, one pair of electrons shares all the same quantum numbers, which is forbidden by the exclusion principle. Instead, one of the electrons is forced into a higher energy state. No separation between three protons exists for which the total energy change of this process is negative—that is, where bonding occurs spontaneously. Similarly, is not covalently bonded under normal conditions, because these atoms have no valence electrons to share. As the atoms are brought together, the wave functions of the core electrons overlap, and due to the exclusion principle, the electrons are forced into a higher energy state. No separation exists for which such a molecule is energetically favorable.
A polyatomic molecule is a molecule made of more than two atoms. Examples range from a simple water molecule to a complex protein molecule. The structures of these molecules can often be understood in terms of covalent bonding and hybridization . Hybridization is a change in the energy structure of an atom in which mixed states (states that can be written as a linear superposition of others) participate in bonding.
To illustrate hybridization, consider the bonding in a simple water molecule, The electron configuration of oxygen is The 1 s and 2 s electrons are in “closed shells” and do not participate in bonding. The remaining four electrons are the valence electrons. These electrons can fill six possible states ( , , , plus spin up and down). The energies of these states are the same, so the oxygen atom can exploit any linear combination of these states in bonding with the hydrogen atoms. These linear combinations (which you learned about in the chapter on atomic structure) are called atomic orbitals, and they are denoted by and The electron charge distributions for these orbitals are given in [link] .
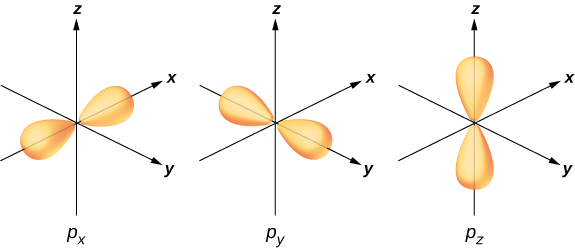
The transformation of the electron wave functions of oxygen to and orbitals in the presence of the hydrogen atoms is an example of hybridization. Two electrons are found in the orbital with paired spins . One electron is found in each of the and orbitals, with unpaired spins. The latter orbitals participate in bonding with the hydrogen atoms. Based on [link] , we expect the bonding angle for to be . However, if we include the effects of repulsion between atoms, the bond angle is . The same arguments can be used to understand the tetrahedral shape of methane and other molecules.
What is the main difference between an ionic bond , a covalent bond , and a van der Waals bond ?
An ionic bond is formed by the attraction of a positive and negative ion. A covalent bond is formed by the sharing of one or more electrons between atoms. A van der Waals bond is formed by the attraction of two electrically polarized molecules.
For the following cases, what type of bonding is expected? (a) KCl molecule; (b) molecule.
Describe three steps to ionic bonding.
1. An electron is removed from one atom. The resulting atom is a positive ion. 2. An electron is absorbed from another atom. The result atom is a negative ion. 3. The positive and negative ions are attracted together until an equilibrium separation is reached.
What prevents a positive and negative ion from having a zero separation?
For the molecule, why must the spins the electron spins be antiparallel?
Bonding is associated with a spatial function that is symmetric under exchange of the two electrons. In this state, the electron density is largest between the atoms. The total function must be antisymmetric (since electrons are fermions), so the spin function must be antisymmetric. In this state, the spins of the electrons are antiparallel.
The electron configuration of carbon is Given this electron configuration, what other element might exhibit the same type of hybridization as carbon?
Potassium chloride (KCl) is a molecule formed by an ionic bond. At equilibrium separation the atoms are apart. Determine the electrostatic potential energy of the atoms.
The electron affinity of Cl is 3.89 eV and the ionization energy of K is 4.34 eV. Use the preceding problem to find the dissociation energy. (Neglect the energy of repulsion.)
The measured energy dissociated energy of KCl is 4.43 eV. Use the results of the preceding problem to determine the energy of repulsion of the ions due to the exclusion principle.

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?