| << Chapter < Page | Chapter >> Page > |
Nuclear physics is an integral part of our everyday lives ( [link] ). Radioactive compounds are used in to identify cancer, study ancient artifacts, and power our cities. Nuclear fusion also powers the Sun, the primary source of energy on Earth. The focus of this chapter is nuclear radiation. In this section, we ask such questions as: How is nuclear radiation used to benefit society? What are its health risks? How much nuclear radiation is the average person exposed to in a lifetime?
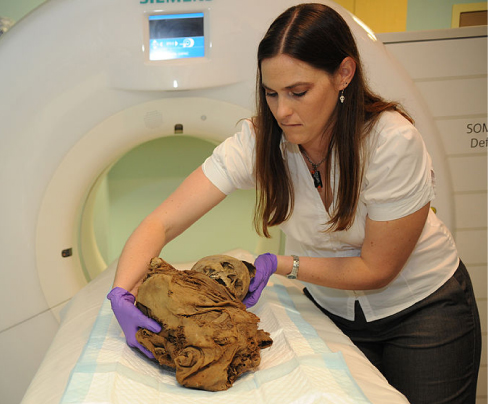
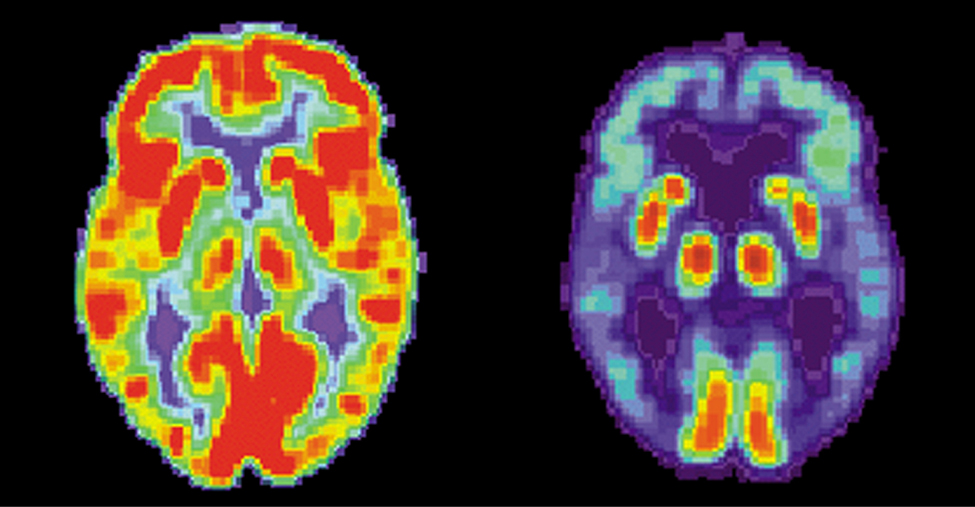
Medical use of nuclear radiation is quite common in today’s hospitals and clinics. One of the most important uses of nuclear radiation is the location and study of diseased tissue. This application requires a special drug called a radiopharmaceutical . A radiopharmaceutical contains an unstable radioactive isotope. When the drug enters the body, it tends to concentrate in inflamed regions of the body. (Recall that the interaction of the drug with the body does not depend on whether a given nucleus is replaced by one of its isotopes, since this interaction is determined by chemical interactions.) Radiation detectors used outside the body use nuclear radiation from the radioisotopes to locate the diseased tissue. Radiopharmaceuticals are called radioactive tags because they allow doctors to track the movement of drugs in the body. Radioactive tags are for many purposes, including the identification of cancer cells in the bones, brain tumors, and Alzheimer’s disease ( [link] ). Radioactive tags are also used to monitor the function of body organs, such as blood flow, heart muscle activity, and iodine uptake in the thyroid gland.
[link] lists some medical diagnostic uses of radiopharmaceuticals, including isotopes and typical activity ( A ) levels. One common diagnostic test uses iodine to image the thyroid, since iodine is concentrated in that organ. Another common nuclear diagnostic is the thallium scan for the cardiovascular system, which reveals blockages in the coronary arteries and examines heart activity. The salt TlCl can be used because it acts like NaCl and follows the blood. Note that [link] lists many diagnostic uses for , where “m” stands for a metastable state of the technetium nucleus. This isotope is used in many compounds to image the skeleton, heart, lungs, and kidneys. About of all radiopharmaceuticals employ because it produces a single, easily identified, 0.142-MeV ray and has a short 6.0-h half-life, which reduces radiation exposure.

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?