| << Chapter < Page | Chapter >> Page > |
| Procedure, Isotope | Activity (mCi), where | Procedure, Isotope | Activity (mCi), where |
|---|---|---|---|
| Brain scan | Thyroid scan | ||
| 7.5 | 0.05 | ||
| (PET) | 50 | 0.07 | |
| Lung scan | Liver scan | ||
| 7.5 | (colloid) | 0.1 | |
| 2 | (colloid) | 2 | |
| Cardiovascular blood pool | Bone scan | ||
| 0.2 | 0.1 | ||
| 2 | 10 | ||
| Cardiovascular arterial flow | Kidney scan | ||
| 3 | 0.1 | ||
| 7.5 | 1.5 |
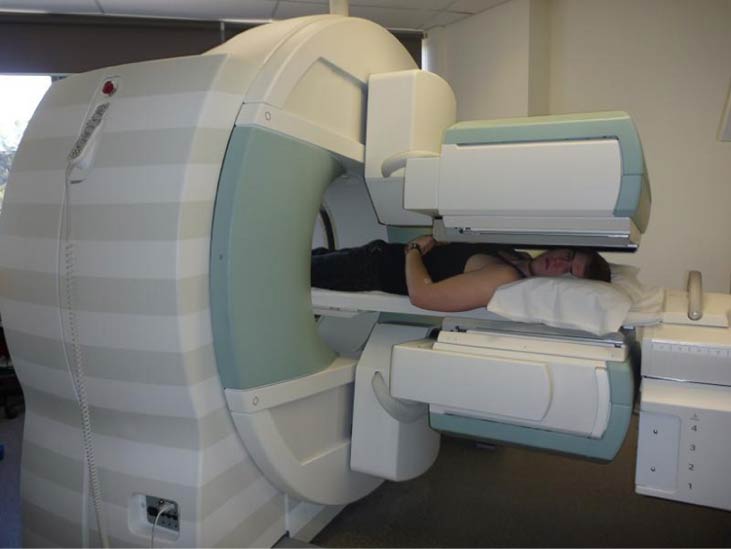
The first radiation detectors produced two-dimensional images, like a photo taken from a camera. However, a circular array of detectors that can be rotated can be used to produce three-dimensional images. This technique is similar to that used in X-ray computed tomography (CT) scans. One application of this technique is called single-photon-emission CT (SPECT) ( [link] ). The spatial resolution of this technique is about 1 cm.
Improved image resolution is achieved by a technique known as positron emission tomography (PET) . This technique use radioisotopes that decay by radiation. When a positron encounters an electron, these particle annihilate to produce two gamma-ray photons. This reaction is represented by
These -ray photons have identical 0.511-MeV energies and move directly away from one another ( [link] ). This easily identified decay signature can be used to identify the location of the radioactive isotope. Examples of -emitting isotopes used in PET include . The nuclei have the advantage of being able to function as tags for natural body compounds. Its resolution of 0.5 cm is better than that of SPECT.
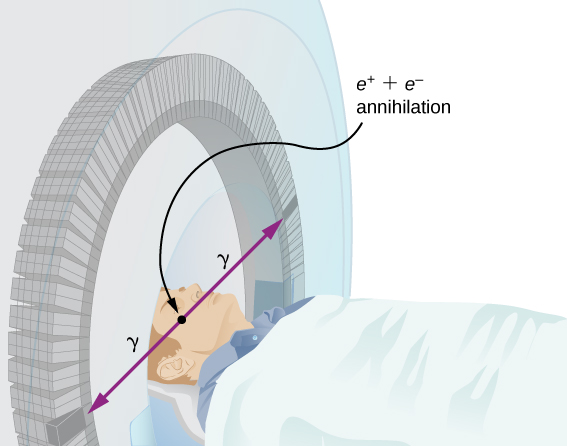
PET scans are especially useful to examine the brain’s anatomy and function. For example, PET scans can be used to monitor the brain’s use of oxygen and water, identify regions of decreased metabolism (linked to Alzheimer’s disease), and locate different parts of the brain responsible for sight, speech, and fine motor activity
Is it a tumor? View an animation of simplified magnetic resonance imaging (MRI) to see if you can tell. Your head is full of tiny radio transmitters (the nuclear spins of the hydrogen nuclei of your water molecules). In an MRI unit, these little radios can be made to broadcast their positions, giving a detailed picture of the inside of your head.
Nuclear radiation can have both positive and negative effects on biological systems. However, it can also be used to treat and even cure cancer. How do we understand these effects? To answer this question, consider molecules within cells, particularly DNA molecules.
Cells have long, double-helical DNA molecules containing chemical codes that govern the function and processes of the cell. Nuclear radiation can alter the structural features of the DNA chain, leading to changes in the genetic code. In human cells, we can have as many as a million individual instances of damage to DNA per cell per day. DNA contains codes that check whether the DNA is damaged and can repair itself. This repair ability of DNA is vital for maintaining the integrity of the genetic code and for the normal functioning of the entire organism. It should be constantly active and needs to respond rapidly. The rate of DNA repair depends on various factors such as the type and age of the cell. If nuclear radiation damages the ability of the cell to repair DNA, the cell can

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?