| << Chapter < Page | Chapter >> Page > |
(a) Draw the pV diagram for the Stirling engine with proper labels.
(b) Fill in the following table.
| Step | W (J) | Q (J) | (J/K) |
|---|---|---|---|
| Step AB | |||
| Step BC | |||
| Step CD | |||
| Step DA | |||
| Complete cycle |
(c) How does the efficiency of the Stirling engine compare to the Carnot engine working within the same two heat reservoirs?
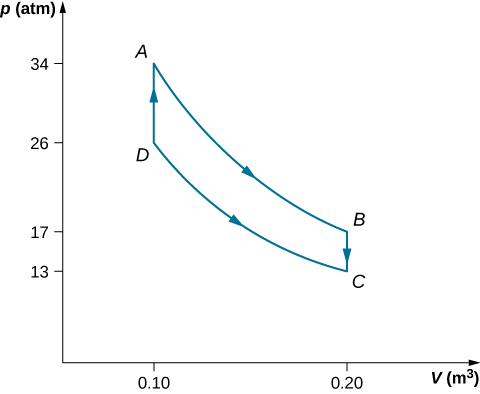
| Step | W (J) | Q (J) | (J/K) |
|---|---|---|---|
| Step AB Isotherm | 2.3 | 2.3 | 0.0057 |
| Step BC Isochoric | 0 | –1.2 | 0.0035 |
| Step CD Isotherm | –1.8 | –1.8 | –0.0059 |
| Step DA Isochoric | 0 | 1.2 | –0.0035 |
| Complete cycle | 0.5 | 0.5 | ~ 0 |
If this were a Carnot engine operating between the same heat reservoirs, its efficiency would be
Therefore, the Carnot engine would have a greater efficiency than the Stirling engine.
The Stirling engine uses compressed air as the working substance, which passes back and forth between two chambers with a porous plug, called the regenerator, which is made of material that does not conduct heat as well. In two of the steps, pistons in the two chambers move in phase.
Does the entropy increase for a Carnot engine for each cycle?
Is it possible for a system to have an entropy change if it neither absorbs nor emits heat during a reversible transition? What happens if the process is irreversible?
Entropy will not change if it is a reversible transition but will change if the process is irreversible.
Two hundred joules of heat are removed from a heat reservoir at a temperature of 200 K. What is the entropy change of the reservoir?
–1 J/K
In an isothermal reversible expansion at , an ideal gas does 20 J of work. What is the entropy change of the gas?
An ideal gas at 300 K is compressed isothermally to one-fifth its original volume. Determine the entropy change per mole of the gas.
–13 J(K mole)
What is the entropy change of 10 g of steam at when it condenses to water at the same temperature?
A metal rod is used to conduct heat between two reservoirs at temperatures respectively. When an amount of heat Q flows through the rod from the hot to the cold reservoir, what is the net entropy change of the rod, the hot reservoir, the cold reservoir, and the universe?
For the Carnot cycle of [link] , what is the entropy change of the hot reservoir, the cold reservoir, and the universe?
A 5.0-kg piece of lead at a temperature of is placed in a lake whose temperature is . Determine the entropy change of (a) the lead piece, (b) the lake, and (c) the universe.
a. –540 J/K; b. 1600 J/K; c. 1100 J/K
One mole of an ideal gas doubles its volume in a reversible isothermal expansion. (a) What is the change in entropy of the gas? (b) If 1500 J of heat are added in this process, what is the temperature of the gas?
One mole of an ideal monatomic gas is confined to a rigid container. When heat is added reversibly to the gas, its temperature changes from (a) How much heat is added? (b) What is the change in entropy of the gas?
a. ; b.
(a) A 5.0-kg rock at a temperature of is dropped into a shallow lake also at from a height of . What is the resulting change in entropy of the universe? (b) If the temperature of the rock is when it is dropped, what is the change of entropy of the universe? Assume that air friction is negligible (not a good assumption) and that is the specific heat of the rock.

Notification Switch
Would you like to follow the 'University physics volume 2' conversation and receive update notifications?