| << Chapter < Page | Chapter >> Page > |
Would you expect to be larger for a gas or a solid? Explain.
There is no change in the internal energy of an ideal gas undergoing an isothermal process since the internal energy depends only on the temperature. Is it therefore correct to say that an isothermal process is the same as an adiabatic process for an ideal gas? Explain your answer.
An adiabatic process has a change in temperature but no heat flow. The isothermal process has no change in temperature but has heat flow.
Does a gas do any work when it expands adiabatically? If so, what is the source of the energy needed to do this work?
A monatomic ideal gas undergoes a quasi-static adiabatic expansion in which its volume is doubled. How is the pressure of the gas changed?
pressure decreased by 0.31 times the original pressure
An ideal gas has a pressure of 0.50 atm and a volume of 10 L. It is compressed adiabatically and quasi-statically until its pressure is 3.0 atm and its volume is 2.8 L. Is the gas monatomic, diatomic, or polyatomic?
Pressure and volume measurements of a dilute gas undergoing a quasi-static adiabatic expansion are shown below. Plot ln p vs. V and determine for this gas from your graph.
| P (atm) | V (L) |
|---|---|
| 20.0 | 1.0 |
| 17.0 | 1.1 |
| 14.0 | 1.3 |
| 11.0 | 1.5 |
| 8.0 | 2.0 |
| 5.0 | 2.6 |
| 2.0 | 5.2 |
| 1.0 | 8.4 |
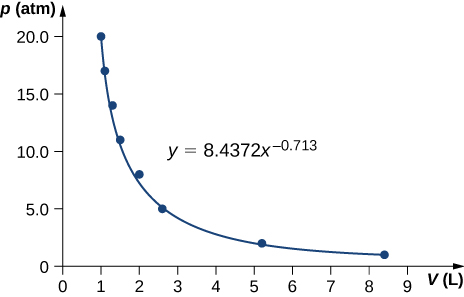 ;
;
An ideal monatomic gas at 300 K expands adiabatically and reversibly to twice its volume. What is its final temperature?
An ideal diatomic gas at 80 K is slowly compressed adiabatically and reversibly to twice its volume. What is its final temperature?
84 K
An ideal diatomic gas at 80 K is slowly compressed adiabatically to one-third its original volume. What is its final temperature?
Compare the charge in internal energy of an ideal gas for a quasi-static adiabatic expansion with that for a quasi-static isothermal expansion. What happens to the temperature of an ideal gas in an adiabatic expansion?
An adiabatic expansion has less work done and no heat flow, thereby a lower internal energy comparing to an isothermal expansion which has both heat flow and work done. Temperature decreases during adiabatic expansion.
The temperature of n moles of an ideal gas changes from to in a quasi-static adiabatic transition. Show that the work done by the gas is given by
A dilute gas expands quasi-statically to three times its initial volume. Is the final gas pressure greater for an isothermal or an adiabatic expansion? Does your answer depend on whether the gas is monatomic, diatomic, or polyatomic?
Isothermal has a greater final pressure and does not depend on the type of gas.
(a) An ideal gas expands adiabatically from a volume of to . If the initial pressure and temperature were and 300 K, respectively, what are the final pressure and temperature of the gas? Use for the gas. (b) In an isothermal process, an ideal gas expands from a volume of to . If the initial pressure and temperature were and 300 K, respectively, what are the final pressure and temperature of the gas?

Notification Switch
Would you like to follow the 'University physics volume 2' conversation and receive update notifications?