| << Chapter < Page | Chapter >> Page > |
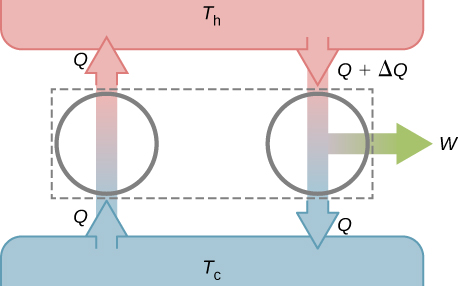
Suppose these two devices are combined as shown in [link] . The net heat removed from the hot reservoir is , no net heat transfer occurs to or from the cold reservoir, and work W is done on some external body. Since , the combination of a perfect refrigerator and a real heat engine is itself a perfect heat engine, thereby contradicting the Kelvin statement. Thus, if the Clausius statement is false, the Kelvin statement must also be false.
Using the second law of thermodynamics, we now prove two important properties of heat engines operating between two heat reservoirs. The first property is that any reversible engine operating between two reservoirs has a greater efficiency than any irreversible engine operating between the same two reservoirs .
The second property to be demonstrated is that all reversible engines operating between the same two reservoirs have the same efficiency . To show this, we start with the two engines D and E of [link] (a), which are operating between two common heat reservoirs at temperatures First, we assume that D is a reversible engine and that E is a hypothetical irreversible engine that has a higher efficiency than D. If both engines perform the same amount of work per cycle, it follows from [link] that . It then follows from the first law that
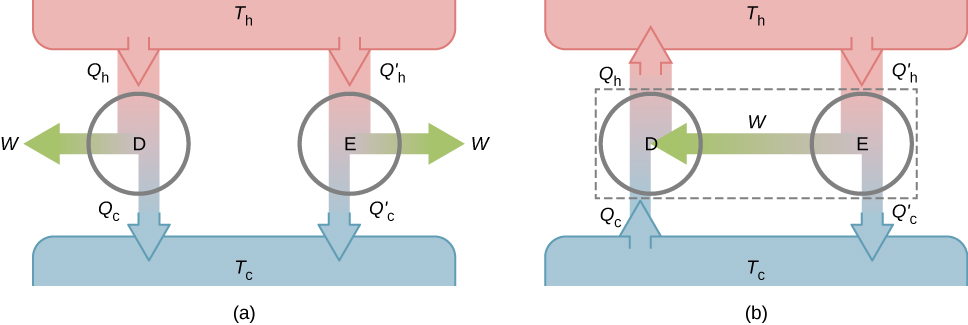
Suppose the cycle of D is reversed so that it operates as a refrigerator, and the two engines are coupled such that the work output of E is used to drive D, as shown in [link] (b). Since and the net result of each cycle is equivalent to a spontaneous transfer of heat from the cold reservoir to the hot reservoir, a process the second law does not allow. The original assumption must therefore be wrong, and it is impossible to construct an irreversible engine such that E is more efficient than the reversible engine D.
Now it is quite easy to demonstrate that the efficiencies of all reversible engines operating between the same reservoirs are equal. Suppose that D and E are both reversible engines. If they are coupled as shown in [link] (b), the efficiency of E cannot be greater than the efficiency of D, or the second law would be violated. If both engines are then reversed, the same reasoning implies that the efficiency of D cannot be greater than the efficiency of E. Combining these results leads to the conclusion that all reversible engines working between the same two reservoirs have the same efficiency.
Check Your Understanding What is the efficiency of a perfect heat engine? What is the coefficient of performance of a perfect refrigerator?
A perfect heat engine would have , which would lead to A perfect refrigerator would need zero work, that is, , which leads to
Check Your Understanding Show that for the hypothetical engine of [link] (b).
From the engine on the right, we have From the refrigerator on the right, we have Thus,
In the text, we showed that if the Clausius statement is false, the Kelvin statement must also be false. Now show the reverse, such that if the Kelvin statement is false, it follows that the Clausius statement is false.
If we combine a perfect engine and a real refrigerator with the engine converting heat Q from the hot reservoir into work to drive the refrigerator, then the heat dumped to the hot reservoir by the refrigerator will be , resulting in a perfect refrigerator transferring heat from the cold reservoir to hot reservoir without any other effect.
Why don’t we operate ocean liners by extracting heat from the ocean or operate airplanes by extracting heat from the atmosphere?
Discuss the practical advantages and disadvantages of heat pumps and electric heating.
Heat pumps can efficiently extract heat from the ground to heat on cooler days or pull heat out of the house on warmer days. The disadvantage of heat pumps are that they are more costly than alternatives, require maintenance, and will not work efficiently when temperature differences between the inside and outside are very large. Electric heating is much cheaper to purchase than a heat pump; however, it may be more costly to run depending on the electric rates and amount of usage.
The energy output of a heat pump is greater than the energy used to operate the pump. Why doesn’t this statement violate the first law of thermodynamics?
Speculate as to why nuclear power plants are less efficient than fossil-fuel plants based on temperature arguments.
A nuclear reactor needs to have a lower temperature to operate, so its efficiency will not be as great as a fossil-fuel plant. This argument does not take into consideration the amount of energy per reaction: Nuclear power has a far greater energy output than fossil fuels.
An ideal gas goes from state to state when it is allowed to expand freely. Is it possible to represent the actual process on a pV diagram? Explain.

Notification Switch
Would you like to follow the 'University physics volume 2' conversation and receive update notifications?