| << Chapter < Page | Chapter >> Page > |
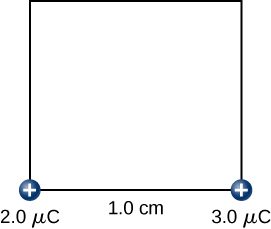
Step 3. While keeping the charges of and fixed in their places, bring in the charge to ( [link] ). The work done in this step is
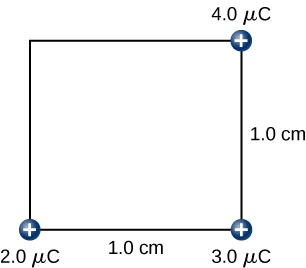
Step 4. Finally, while keeping the first three charges in their places, bring the charge to ( [link] ). The work done here is
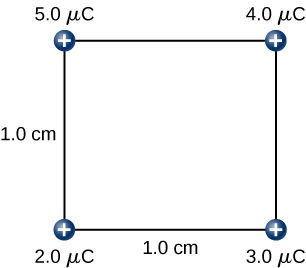
Hence, the total work done by the applied force in assembling the four charges is equal to the sum of the work in bringing each charge from infinity to its final position:
Check Your Understanding Is the electrical potential energy of two point charges positive or negative if the charges are of the same sign? Opposite signs? How does this relate to the work necessary to bring the charges into proximity from infinity?
positive, negative, and these quantities are the same as the work you would need to do to bring the charges in from infinity
Note that the electrical potential energy is positive if the two charges are of the same type, either positive or negative, and negative if the two charges are of opposite types. This makes sense if you think of the change in the potential energy as you bring the two charges closer or move them farther apart. Depending on the relative types of charges, you may have to work on the system or the system would do work on you, that is, your work is either positive or negative. If you have to do positive work on the system (actually push the charges closer), then the energy of the system should increase. If you bring two positive charges or two negative charges closer, you have to do positive work on the system, which raises their potential energy. Since potential energy is proportional to 1/ r , the potential energy goes up when r goes down between two positive or two negative charges.
On the other hand, if you bring a positive and a negative charge nearer, you have to do negative work on the system (the charges are pulling you), which means that you take energy away from the system. This reduces the potential energy. Since potential energy is negative in the case of a positive and a negative charge pair, the increase in 1/ r makes the potential energy more negative, which is the same as a reduction in potential energy.
The result from [link] may be extended to systems with any arbitrary number of charges. In this case, it is most convenient to write the formula as
The factor of 1/2 accounts for adding each pair of charges twice.
Would electric potential energy be meaningful if the electric field were not conservative?
No. We can only define potential energies for conservative fields.
Why do we need to be careful about work done on the system versus work done by the system in calculations?
Does the order in which we assemble a system of point charges affect the total work done?
No, though certain orderings may be simpler to compute.
Consider a charge fixed at a site with another charge (charge , mass moving in the neighboring space. (a) Evaluate the potential energy of when it is 4.0 cm from (b) If starts from rest from a point 4.0 cm from what will be its speed when it is 8.0 cm from ? ( Note: is held fixed in its place.)
a.
b.
Two charges and are placed symmetrically along the x -axis at . Consider a charge of charge and mass 10.0 mg moving along the y -axis. If starts from rest at what is its speed when it reaches
To form a hydrogen atom, a proton is fixed at a point and an electron is brought from far away to a distance of the average distance between proton and electron in a hydrogen atom. How much work is done?
(a) What is the average power output of a heart defibrillator that dissipates 400 J of energy in 10.0 ms? (b) Considering the high-power output, why doesn’t the defibrillator produce serious burns?

Notification Switch
Would you like to follow the 'University physics volume 2' conversation and receive update notifications?