| << Chapter < Page | Chapter >> Page > |
If the vapor pressure of the liquid is greater than the total ambient pressure, including that of any air (or other gas), the liquid evaporates rapidly; in other words, it boils. Thus, the boiling point of a liquid at a given pressure is the temperature at which its vapor pressure equals the ambient pressure. Liquid and gas phases are in equilibrium at the boiling temperature ( [link] ). If a substance is in a closed container at the boiling point, then the liquid is boiling and the gas is condensing at the same rate without net change in their amounts.
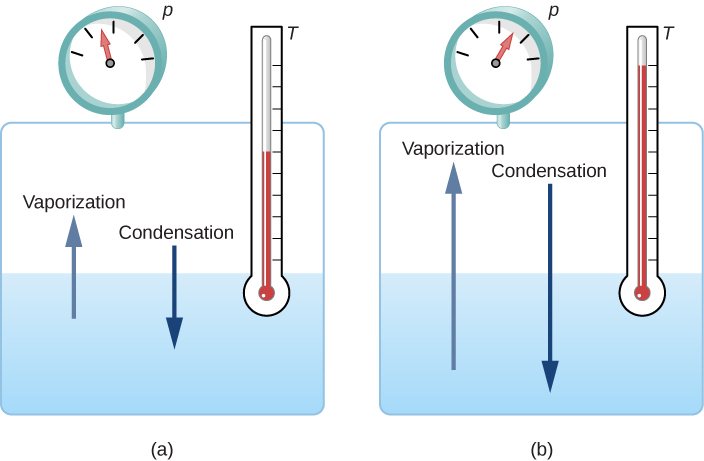
For water, is the boiling point at 1.00 atm, so water and steam should exist in equilibrium under these conditions. Why does an open pot of water at boil completely away? The gas surrounding an open pot is not pure water: it is mixed with air. If pure water and steam are in a closed container at and 1.00 atm, they will coexist—but with air over the pot, there are fewer water molecules to condense, and water boils away. Another way to see this is that at the boiling point, the vapor pressure equals the ambient pressure. However, part of the ambient pressure is due to air, so the pressure of the steam is less than the vapor pressure at that temperature, and evaporation continues. Incidentally, the equilibrium vapor pressure of solids is not zero, a fact that accounts for sublimation.
Check Your Understanding Explain why a cup of water (or soda) with ice cubes stays at even on a hot summer day.
The ice and liquid water are in thermal equilibrium, so that the temperature stays at the freezing temperature as long as ice remains in the liquid. (Once all of the ice melts, the water temperature will start to rise.)
So far, we have discussed heat transfers that cause temperature change. However, in a phase transition, heat transfer does not cause any temperature change.
For an example of phase changes, consider the addition of heat to a sample of ice at ( [link] ) and atmospheric pressure. The temperature of the ice rises linearly, absorbing heat at a constant rate of until it reaches Once at this temperature, the ice begins to melt and continues until it has all melted, absorbing 333 kJ/kg of heat. The temperature remains constant at during this phase change. Once all the ice has melted, the temperature of the liquid water rises, absorbing heat at a new constant rate of At the water begins to boil. The temperature again remains constant during this phase change while the water absorbs 2256 kJ/kg of heat and turns into steam. When all the liquid has become steam, the temperature rises again, absorbing heat at a rate of . If we started with steam and cooled it to make it condense into liquid water and freeze into ice, the process would exactly reverse, with the temperature again constant during each phase transition.

Notification Switch
Would you like to follow the 'University physics volume 2' conversation and receive update notifications?