| << Chapter < Page | Chapter >> Page > |
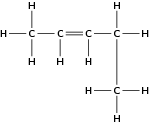
Give the IUPAC name for each of the following alkenes:
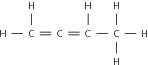
The properties of the alkenes are very similar to those of the alkanes, except that the alkenes are more reactive because they are unsaturated. As with the alkanes, compounds that have four or less carbon atoms are gases at room temperature, while those with five or more carbon atoms are liquids.
Alkenes can undergo addition reactions because they are unsaturated. They readily react with hydrogen, water and the halogens. The double bond is broken and a single, saturated bond is formed. A new group is then added to one or both of the carbon atoms that previously made up the double bond. The following are some examples:
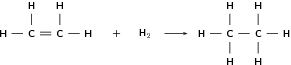
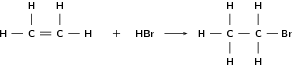
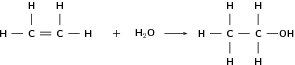
| Formula | Name | Melting point ( C) | Boiling point ( C) |
| C H | Butane | -138 | -0.5 |
| C H | Pentane | -130 | 36 |
| C H | Hexane | -95 | 69 |
| C H | Butene | -185 | -6 |
| C H | Pentene | -138 | 30 |
| C H | Hexene | -140 | 63 |
In the alkynes, there is at least one triple bond between two of the carbon atoms. They are unsaturated compounds and are therefore highly reactive. Their general formula is C H . The simplest alkyne is ethyne ( [link] ), also known as acetylene. Many of the alkynes are used to synthesise other chemical products.

The raw materials that are needed to make acetylene are calcium carbonate and coal. Acetylene can be produced through the following reactions:
An important use of acetylene is in oxyacetylene gas welding. The fuel gas burns with oxygen in a torch. An incredibly high heat is produced, and this is enough to melt metal.
The same rules will apply as for the alkanes and alkenes, except that the suffix of the name will now be -yne.
Give the IUPAC name for the following compound:
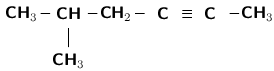
There is a triple bond between two of the carbon atoms, so this compound is an alkyne. The suffix will be -yne. The triple bond is at the second carbon, so the suffix will in fact be 2-yne.
If we count the carbons in a straight line, there are six. The prefix of the compound's name will be 'hex'.
In this example, you will need to number the carbons from right to left so that the triple bond is between carbon atoms with the lowest numbers.
There is a methyl (CH ) group attached to the fifth carbon (remember we have numbered the carbon atoms from right to left).
If we follow this order, the name of the compound is 5-methyl-hex-2-yne .
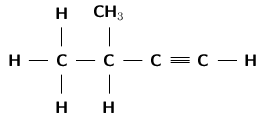
Give the IUPAC name for each of the following organic compounds.

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 12 physical science' conversation and receive update notifications?