| << Chapter < Page | Chapter >> Page > |
In a lead-acid battery , each cell consists of electrodes of lead (Pb) and lead (IV) oxide (PbO ) in an electrolyte of sulfuric acid (H SO ). When the battery discharges, both electrodes turn into lead (II) sulphate (PbSO ) and the electrolyte loses sulfuric acid to become mostly water.
The chemical half reactions that take place at the anode and cathode when the battery is discharging are as follows:
Anode (oxidation): (E = -0.356 V)
Cathode (reduction): (E = 1.685 V)
The overall reaction is as follows:
The emf of the cell is calculated as follows:
EMF = E (cathode)- E (anode)
EMF = +1.685 V - (-0.356 V)
EMF = +2.041 V
Since most batteries consist of six cells, the total voltage of the battery is approximately 12 V.
One of the important things about a lead-acid battery is that it can be recharged . The recharge reactions are the reverse of those when the battery is discharging.
The lead-acid battery is made up of a number of plates that maximise the surface area on which chemical reactions can take place. Each plate is a rectangular grid, with a series of holes in it. The holes are filled with a mixture of lead and sulfuric acid. This paste is pressed into the holes and the plates are then stacked together, with suitable separators between them. They are then placed in the battery container, after which acid is added ( [link] ).
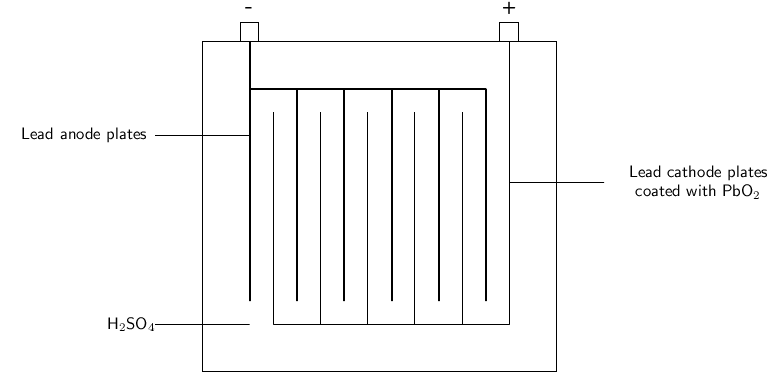
Lead-acid batteries have a number of applications. They can supply high surge currents, are relatively cheap, have a long shelf life and can be recharged. They are ideal for use in cars, where they provide the high current that is needed by the starter motor. They are also used in forklifts and as standby power sources in telecommunication facilities, generating stations and computer data centres. One of the disadvantages of this type of battery is that the battery's lead must be recycled so that the environment doesn't become contaminated. Also, sometimes when the battery is charging, hydrogen gas is generated at the cathode and this can cause a small explosion if the gas comes into contact with a spark.
A simplified diagram of a zinc-carbon cell is shown in [link] .
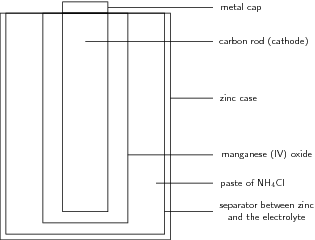
A zinc-carbon cell is made up of an outer zinc container, which acts as the anode . The cathode is the central carbon rod, surrounded by a mixture of carbon and manganese (IV) oxide (MnO ). The electrolyte is a paste of ammonium chloride (NH Cl). A fibrous fabric separates the two electrodes, and a brass pin in the centre of the cell conducts electricity to the outside circuit.
The paste of ammonium chloride reacts according to the following half-reaction:
The manganese(IV) oxide in the cell removes the hydrogen produced above, according to the following reaction:
The combined result of these two reactions can be represented by the following half reaction, which takes place at the cathode:

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 12 physical science' conversation and receive update notifications?