| << Chapter < Page | Chapter >> Page > |
In certain types of radioactive nuclei that have too many neutrons, a neutron may be converted into a proton, an electron and another particle called a neutrino. The high energy electrons that are released in this way are the - particles. This process can occur for an isolated neutron.
In + decay, energy is used to convert a proton into a neutron(n), a positron (e+) and a neutrino ( e):
So, unlike -, + decay cannot occur in isolation, because it requires energy, the mass of the neutron being greater than the mass of the proton. + decay can only happen inside nuclei when the value of the binding energy of the mother nucleus is less than that of the daughter nucleus. The difference between these energies goes into the reaction ofconverting a proton into a neutron, a positron and a neutrino and into the kinetic energy of these particles.
The diagram below shows what happens during decay:
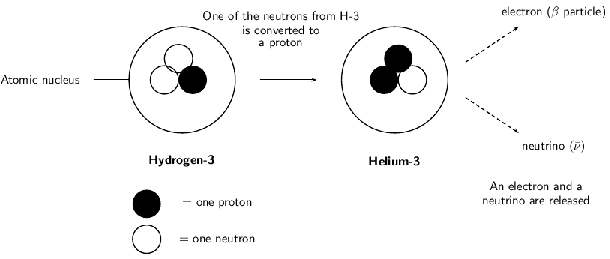
During beta decay, the number of neutrons in the atom decreases by one, and the number of protons increases by one. Since the number of protons before and after the decay is different, the atom has changed into a different element. In [link] , Hydrogen has become Helium. The beta decay of the Hydrogen-3 atom can be represented as follows:
Due to the radioactive processes inside the sun, each 1 patch of the earth receives 70 billion (70 ) neutrinos each second! Luckily neutrinos only interact very weakly so they do not harm our bodies when billions of them pass through us every second.
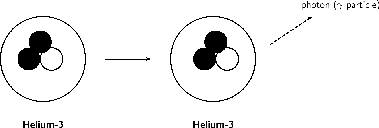
When particles inside the nucleus collide during radioactive decay, energy is released. This energy can leave the nucleus in the form of waves of electromagnetic energy called gamma rays. Gamma radiation is part of the electromagnetic spectrum, just like visible light. However, unlike visible light, humans cannot see gamma rays because they are at a much higher frequency and a higher energy. Gamma radiation has no mass or charge. This type of radiation is able to penetrate most common substances, including metals. Only substance with high atomic masses (like lead) and high densities (like concrete or granite) are effective at absorbing gamma rays.
Gamma decay occurs if the nucleus is at a very high energy state. Since gamma rays are part of the electromagnetic spectrum, they can be thought of as waves or particles. Therefore in gamma decay, we can think of a ray or a particle (called a photon) being released. The atomic number and atomic mass remain unchanged.
[link] summarises and compares the three types of radioactive decay that have been discussed.
| Type of decay | Particle/ray released | Change in element | Penetration power |
| Alpha ( ) | particle (2 protons and 2 neutrons) | Yes | Low |
| Beta ( ) | particle (electron or positron) | Yes | Medium |
| Gamma ( ) | ray (electromagnetic energy) | No | High |
Types of decay
The isotope Am undergoes radioactive decay and loses two alpha particles.
One particle consists of two protons and two neutrons. Since two particles are released, the total number of protons lost is four and the total number of neutrons lost is also four.
The element that has Z = 91 is Protactinium (Pa).
Pa
+ 4 protons + 4 neutrons
In groups of 3-4, discuss the following questions:

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 11 physical science' conversation and receive update notifications?