| << Chapter < Page | Chapter >> Page > |
[link] is a schematic representation of the two fundamental parts of any voltage source. The emf (represented by a script E in the figure) and internal resistance are in series. The smaller the internal resistance for a given emf, the more current and the more power the source can supply.
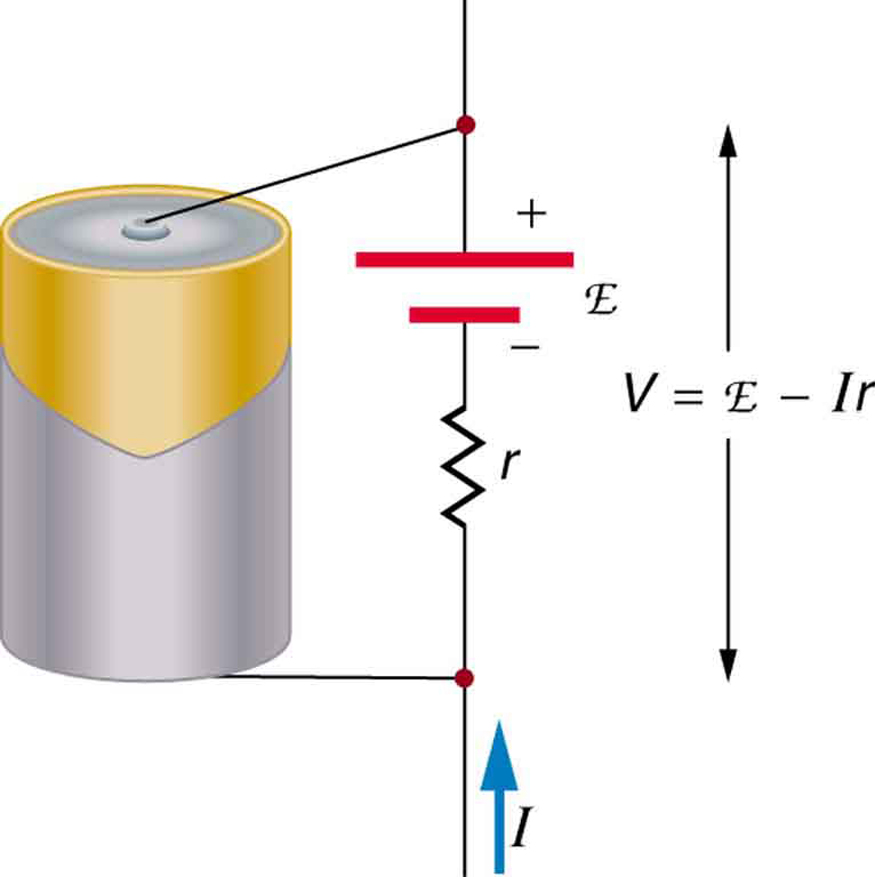
The internal resistance can behave in complex ways. As noted, increases as a battery is depleted. But internal resistance may also depend on the magnitude and direction of the current through a voltage source, its temperature, and even its history. The internal resistance of rechargeable nickel-cadmium cells, for example, depends on how many times and how deeply they have been depleted.
Various types of batteries are available, with emfs determined by the combination of chemicals involved. We can view this as a molecular reaction (what much of chemistry is about) that separates charge.
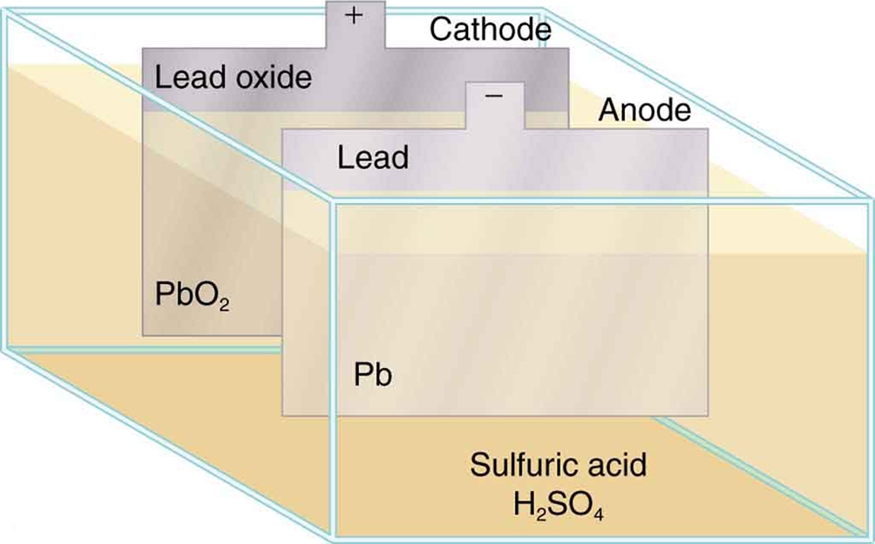
The lead-acid battery used in cars and other vehicles is one of the most common types. A single cell (one of six) of this battery is seen in [link] . The cathode (positive) terminal of the cell is connected to a lead oxide plate, while the anode (negative) terminal is connected to a lead plate. Both plates are immersed in sulfuric acid, the electrolyte for the system.
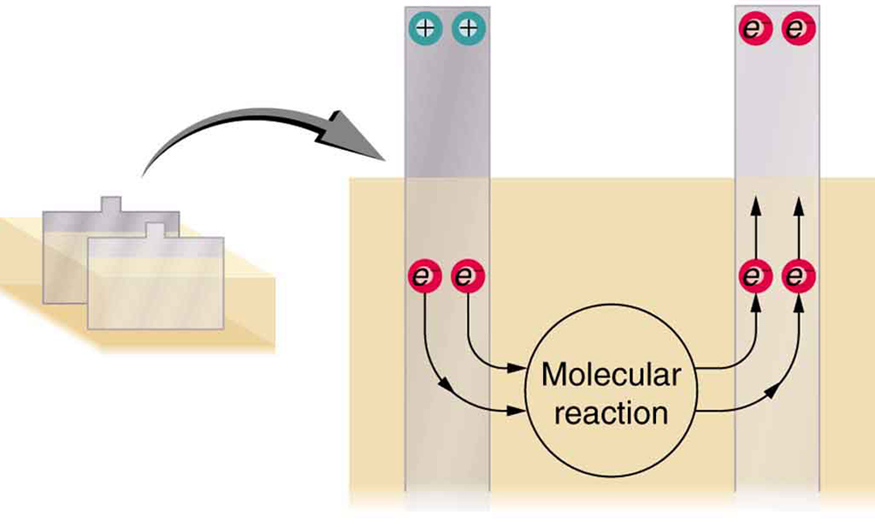
The details of the chemical reaction are left to the reader to pursue in a chemistry text, but their results at the molecular level help explain the potential created by the battery. [link] shows the result of a single chemical reaction. Two electrons are placed on the anode, making it negative, provided that the cathode supplied two electrons. This leaves the cathode positively charged, because it has lost two electrons. In short, a separation of charge has been driven by a chemical reaction.
Note that the reaction will not take place unless there is a complete circuit to allow two electrons to be supplied to the cathode. Under many circumstances, these electrons come from the anode, flow through a resistance, and return to the cathode. Note also that since the chemical reactions involve substances with resistance, it is not possible to create the emf without an internal resistance.

Notification Switch
Would you like to follow the 'College physics (engineering physics 2, tuas)' conversation and receive update notifications?