| << Chapter < Page | Chapter >> Page > |
The fact that nuclear forces are very strong is responsible for the very large energies emitted in nuclear decay. During decay, the forces do work, and since work is force times the distance ( ), a large force can result in a large emitted energy. In fact, we know that there are two distinct nuclear forces because of the different types of nuclear decay—the strong nuclear force is responsible for decay, while the weak nuclear force is responsible for decay.
The many stable and unstable nuclei we have explored, and the hundreds we have not discussed, can be arranged in a table called the chart of the nuclides , a simplified version of which is shown in [link] . Nuclides are located on a plot of versus . Examination of a detailed chart of the nuclides reveals patterns in the characteristics of nuclei, such as stability, abundance, and types of decay, analogous to but more complex than the systematics in the periodic table of the elements.
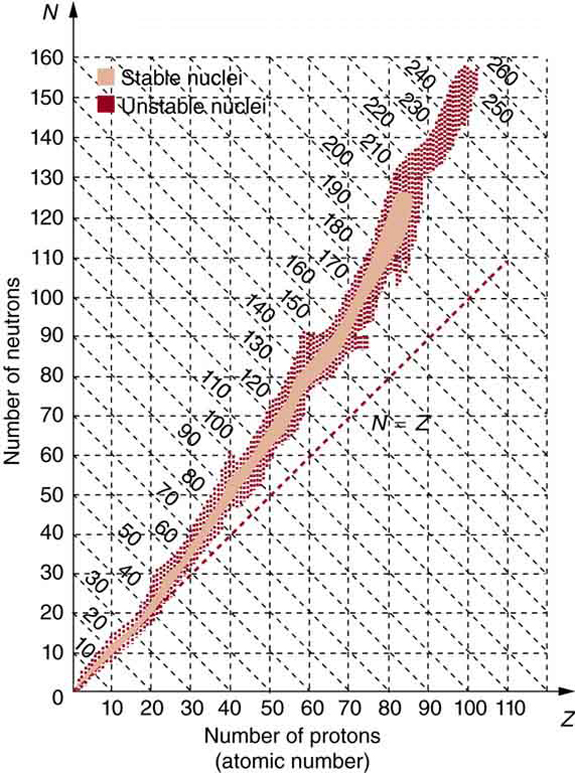
In principle, a nucleus can have any combination of protons and neutrons, but [link] shows a definite pattern for those that are stable. For low-mass nuclei, there is a strong tendency for and to be nearly equal. This means that the nuclear force is more attractive when . More detailed examination reveals greater stability when and are even numbers—nuclear forces are more attractive when neutrons and protons are in pairs. For increasingly higher masses, there are progressively more neutrons than protons in stable nuclei. This is due to the ever-growing repulsion between protons. Since nuclear forces are short ranged, and the Coulomb force is long ranged, an excess of neutrons keeps the protons a little farther apart, reducing Coulomb repulsion. Decay modes of nuclides out of the region of stability consistently produce nuclides closer to the region of stability. There are more stable nuclei having certain numbers of protons and neutrons, called magic numbers . Magic numbers indicate a shell structure for the nucleus in which closed shells are more stable. Nuclear shell theory has been very successful in explaining nuclear energy levels, nuclear decay, and the greater stability of nuclei with closed shells. We have been producing ever-heavier transuranic elements since the early 1940s, and we have now produced the element with . There are theoretical predictions of an island of relative stability for nuclei with such high s.

The weak and strong nuclear forces are basic to the structure of matter. Why we do not experience them directly?
Define and make clear distinctions between the terms neutron, nucleon, nucleus, nuclide, and neutrino.
What are isotopes? Why do different isotopes of the same element have similar chemistries?
Verify that a mass of water at normal density would make a cube 60 km on a side, as claimed in [link] . (This mass at nuclear density would make a cube 1.0 m on a side.)
Find the length of a side of a cube having a mass of 1.0 kg and the density of nuclear matter, taking this to be .
What is the radius of an particle?
Find the radius of a nucleus. is a manufactured nuclide that is used as a power source on some space probes.
(a) Calculate the radius of , one of the most tightly bound stable nuclei.
(b) What is the ratio of the radius of to that of , one of the largest nuclei ever made? Note that the radius of the largest nucleus is still much smaller than the size of an atom.
(a)
(b)
The unified atomic mass unit is defined to be . Verify that this amount of mass converted to energy yields 931.5 MeV. Note that you must use four-digit or better values for and .
What is the ratio of the velocity of a particle to that of an particle, if they have the same nonrelativistic kinetic energy?
If a 1.50-cm-thick piece of lead can absorb 90.0% of the rays from a radioactive source, how many centimeters of lead are needed to absorb all but 0.100% of the rays?
The detail observable using a probe is limited by its wavelength. Calculate the energy of a -ray photon that has a wavelength of , small enough to detect details about one-tenth the size of a nucleon. Note that a photon having this energy is difficult to produce and interacts poorly with the nucleus, limiting the practicability of this probe.
(a) Show that if you assume the average nucleus is spherical with a radius , and with a mass of u, then its density is independent of .
(b) Calculate that density in and , and compare your results with those found in [link] for .
What is the ratio of the velocity of a 5.00-MeV ray to that of an particle with the same kinetic energy? This should confirm that s travel much faster than s even when relativity is taken into consideration. (See also [link] .)
19.3 to 1
(a) What is the kinetic energy in MeV of a ray that is traveling at ? This gives some idea of how energetic a ray must be to travel at nearly the same speed as a ray. (b) What is the velocity of the ray relative to the ray?

Notification Switch
Would you like to follow the 'Basic physics for medical imaging' conversation and receive update notifications?