| << Chapter < Page | Chapter >> Page > |
The only stable chalcogenides of aluminum are Al 2 S 3 (white), Al 2 Se 3 (grey), and Al 2 Te 3 (dark grey). They are each prepared by the direct reaction of the elements (100 °C) and hydrolyze rapidly in aqueous solution, [link] . All the chalcogenides have a hexagonal ZnS structure in which 2 / 3 of the metal sites are occupied.
The chalcogenides of gallium and indium are more numerous than those of aluminum, and are listed in [link] and [link] along with selected physical properties.
| Compound | Structural type | Crystallographic system | Cell parameters (Å, °) | Band Gap (eV) a |
| GaS | Hexagonal | a = 3.587, c = 15.492 | 3.05 (dir.), 2.593 (ind.) | |
| GaS | ZnS or NaCl | Cubic | a = 5.5 | 4.0 (opt.) |
| β-GaSe | GaS | Hexagonal | a = 3.742, c = 15.919 | 2.103 (dir.), 2.127 (ind.) |
| γ-GaSe | GaS | Rhombohedral | a = 3.755, c = 23.92 | |
| δ-GaSe | GaS | Hexagonal | a = 3.755, c = 31.99 | |
| β-GaTe | GaS | Hexagonal | a = 4.06, c = 16.96 | |
| GaTe | GaS | Monoclinic | a = 17.44, b = 4.077, c = 10.456, β = 104.4 | 1.799 (dir.) |
| α-Ga 2 S 3 | Wurtzite | Cubic | a = 5.181 | |
| α-Ga 2 S 3 | Wurtzite | Monoclinic | a = 12.637, b = 6.41, c = 7.03, β = 131.08 | 3.438 (opt.) |
| β-Ga 2 S 3 | Defect wurtzite | Hexagonal | a = 3.685, c = 6.028 | 2.5 - 2.7 (opt.) |
| α-Ga 2 Se 3 | Sphalerite | Cubic | a = 5.429 | 2.1 (dir.), 2.04 (ind.) |
| α-Ga 2 Te 3 | Sphalerite | Cubic | a = 5.886 | 1.22 (opt.) |
| Compound | Structural type | Crystallographic system | Cell parameters (Å, °) | Band gap (eV) a |
| β-InS | GaS | Orthorhombic | a = 3.944, b = 4.447, c = 10.648 | 2.58 (dir.), 2.067 (ind.) |
| InS b | Hg 2 Cl 2 | Tetragonal | ||
| InSe | GaS | Rhombohedral | a = 4.00, c = 25.32 | 1.3525 (dir.), 1.32 (ind.) |
| β-InSe | GaS | Hexagonal | a = 4.05, c = 16.93 | |
| InTe | TlSe | Tetragonal | a = 8.437, c = 7.139 | Metallic |
| InTe b | NaCl | Cubic | a = 6.18 | |
| α-In 2 S 3 | γ-Al 2 O 3 | Cubic | a = 5.36 | |
| β-In 2 S 3 | Spinel | Tetragonal | a = 7.618, c = 32.33 | 2.03 (dir.), 1.1 (ind.) |
| α-In 2 Se 3 | Defect wurtzite | Hexagonal | a = 16.00, c = 19.24 | |
| β-In 2 Se 3 | Defect wurtzite | Rhombohedral | a = 4.025, c = 19.222 | 1.2 - 1.5 (ind.) |
| α-In 2 Te 3 | Sphalerite | Cubic | a = 6.158 | 0.92 - 1.15 (opt.) |
| In 6 S 7 | Monoclinic | a = 9.090, b = 3.887, c = 17.705, β = 108.20 | 0.89 (dir.), 0.7 (ind.) | |
| In 6 Se 7 | In 6 S 7 | Monoclinic | a = 9.430, b = 4.063, c = 18.378, β = 109.34 | 0.86 (dir.), 0.34 (ind.) |
| In 4 Se 3 | Orthorhombic | a = 15.297, b = 12.308, c = 4.081 | 0.64 (dir.) | |
| In 4 Te 3 | In 4 Se 3 | Orthorhombic | a = 15.630, b = 12.756, c = 4.441 | 0.48 (dir.) |
The hexagonal β -form of Ga 2 S 3 is isostructural with the aluminum analogue; however, while the α-phase was proposed to be hexagonal it was later shown to be monoclinic. A cubic α-phase has been reported. Cubic Sphalerite structures are found for Ga 2 Se 3 , Ga 2 Te 3 , and In 2 Te 3 , in which the structure is based on a cubic close packing of the chalcogenides and the metal atoms occupying 1 / 3 of the tetrahedral sites. These structures are all formed with rapid crystallization; slow crystallization and/or thermal annealing leads to ordering and the formation of more complex structures. The indium sulfides, and selenides derivatives are spinel (γ-Al 2 O 3 ), and defect Würtzite, respectively.
Unlike the chalcogenides of aluminum, those of gallium and indium also form subvalent compounds, i.e., those in which the metal is formally of an oxidation state less than +3. Of these subvalent chalcogenides the (formally) divalent materials are of the most interest. The thermodynamically stable phase of GaS has a hexagonal layer structure ( [link] ) with Ga-Ga bonds (2.48 Å). The compound can, therefore, be considered as an example of Ga(II). Each Ga is coordinated by three sulfur atoms and one gallium, and the sequence of layers along the z -axis is ... S-Ga-Ga-S ... S-Ga-Ga-S ... .
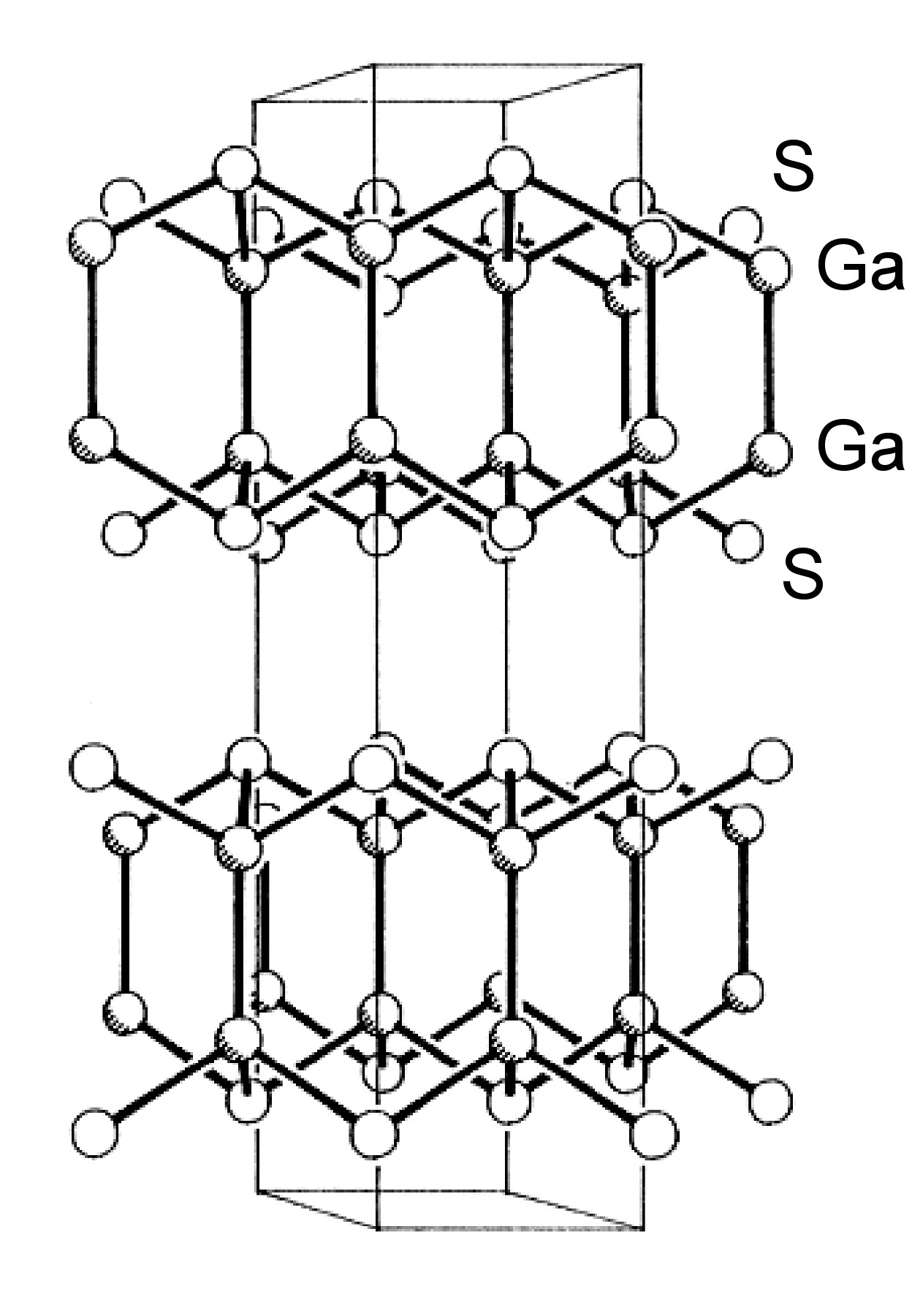
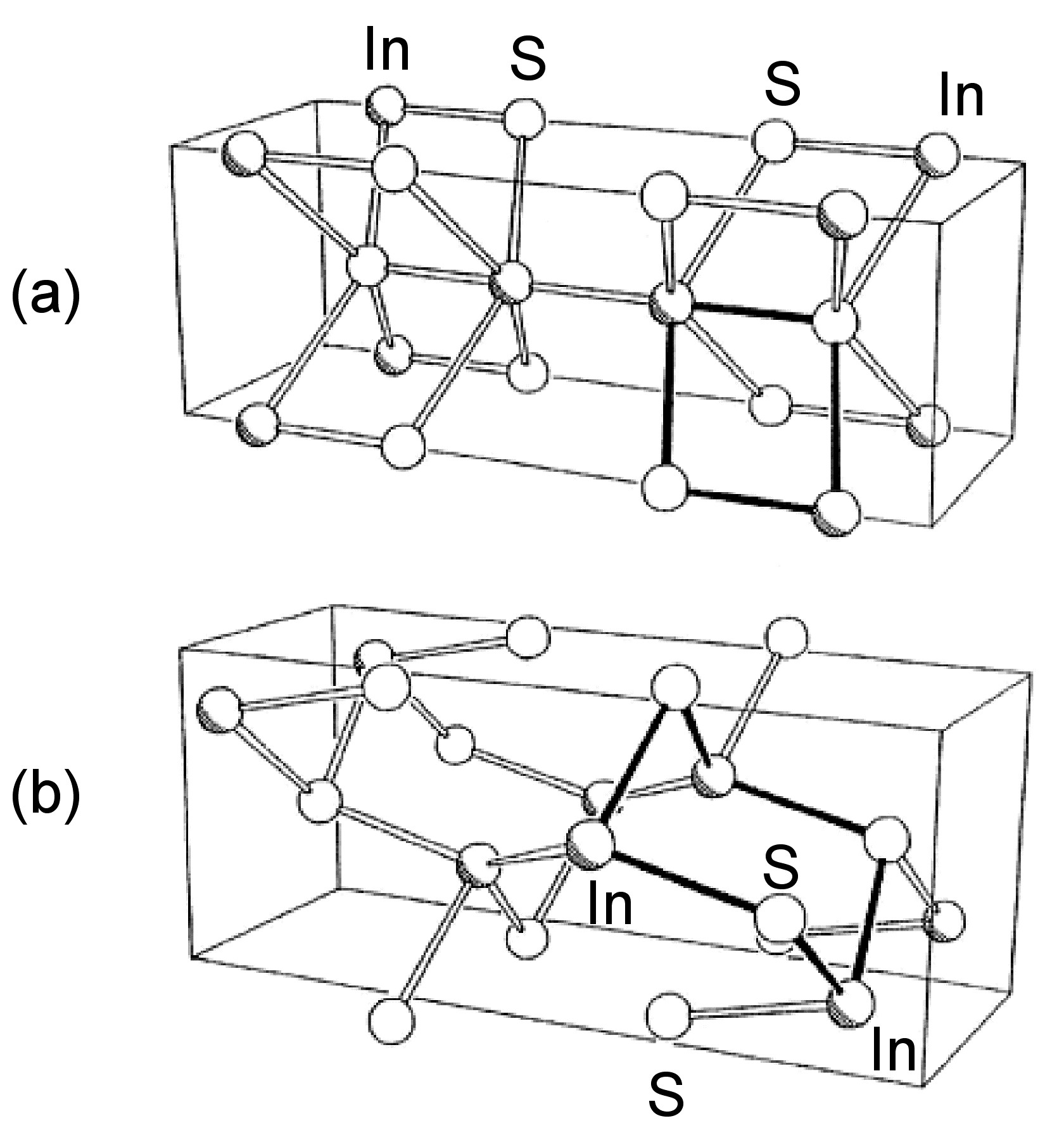
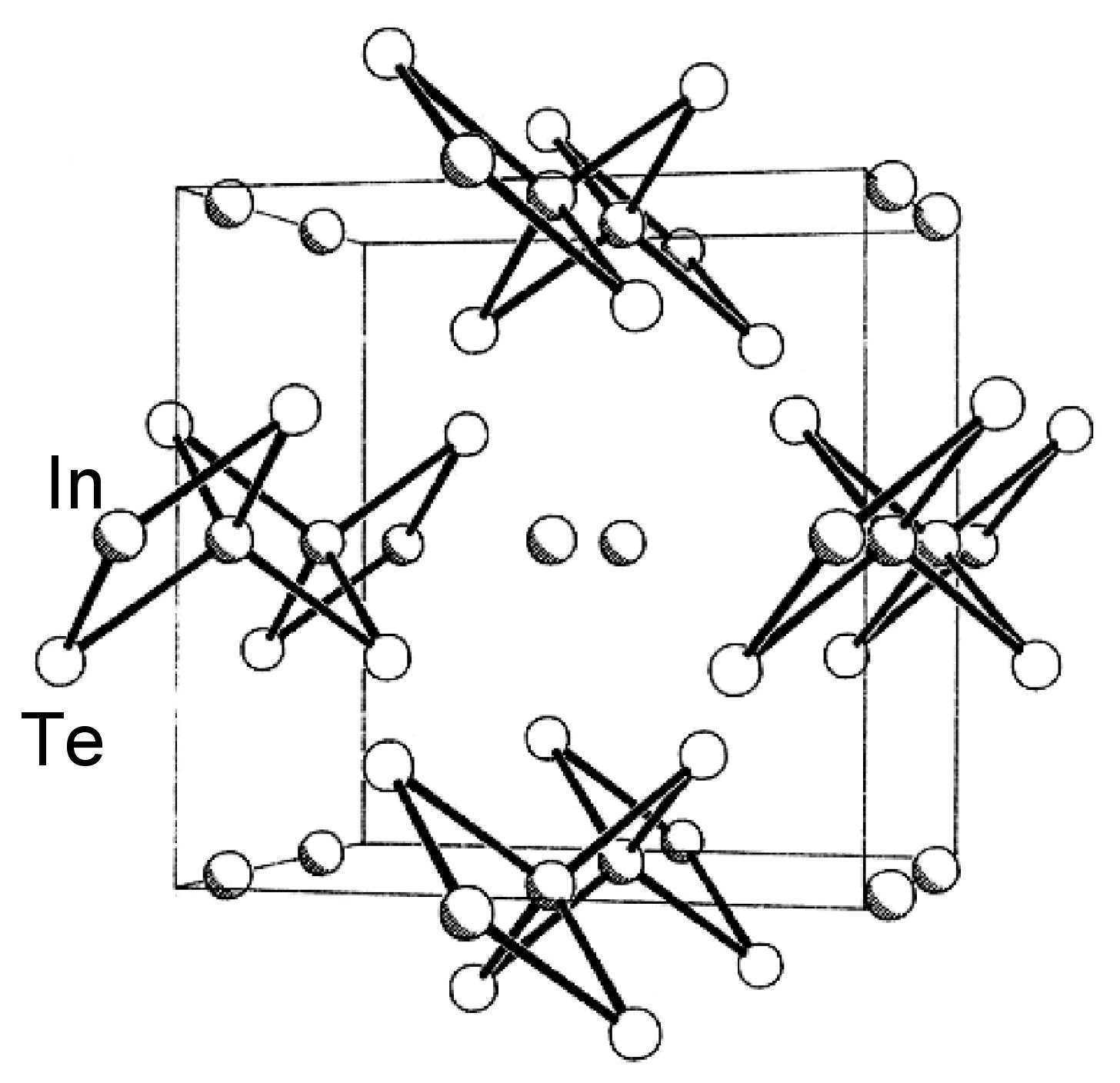
The structures of β-GaSe, and β-InSe are similar to hexagonal GaS. The layered structure of GaTe is similar in that it consists of ... TeGaGaTe ... layers, but is monoclinic, while InS is found in both a (high pressure) tetragonal phase ( [link] a) as well as an orthorhombic phase ( [link] b). By contrast to these M-M bonded layered compounds InTe ( [link] ) has a structure formalized as In(I)[In(III)Te 2 ]; each In(III) is tetrahedrally coordinated to four Te and these tetrahedra are linked via shared edges; the In(I) centers lying between these chains.
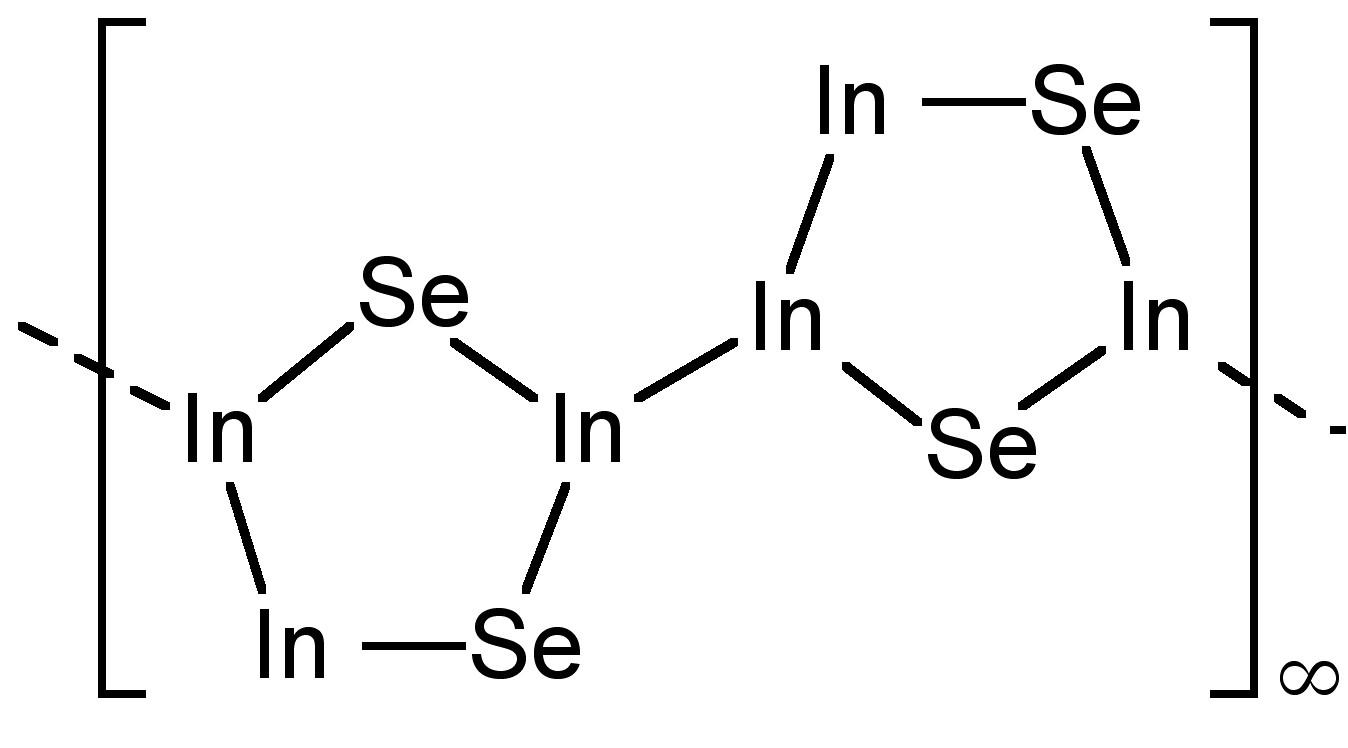
Further sub-chalcogenides are known for indium, e.g.; In 4 Se 3 , which contains [In(III) 3 Se 2 ] 5+ groups ( [link] ). While the formally In(I) molecule In 2 S has been detected in the gas phase, it is actually a mixture of In and InS in the solid state.

Notification Switch
Would you like to follow the 'Chemistry of the main group elements' conversation and receive update notifications?