| << Chapter < Page | Chapter >> Page > |
In the previous section, we focused our attention on the electron configuration of neutral atoms. In a neutral atom, the number of protons is the same as the number of electrons. But what happens if an atom gains or loses electrons? Does it mean that the atom will still be part of the same element?
A change in the number of electrons of an atom does not change the type of atom that it is. However, the charge of the atom will change. If electrons are added, then the atom will become more negative . If electrons are taken away, then the atom will become more positive . The atom that is formed in either of these cases is called an ion . Put simply, an ion is a charged atom.
An ion is a charged atom. A positively charged ion is called a cation e.g. , and a negatively charged ion is called an anion e.g. . The charge on an ion depends on the number of electrons that have been lost or gained.
But how do we know how many electrons an atom will gain or lose? Remember what we said about stability? We said that all atoms are trying to get a full outer shell. For the elements on the left hand side of the periodic table the easiest way to do this is to lose electrons and for the elements on the right of the periodic table the easiest way to do this is to gain electrons. So the elements on the left of the periodic table will form cations and the elements on the right hand side of the periodic table will form anions. By doing this the elements can be in the most stable electronic configuration and so be as stable as the noble gases.
Look at the following examples. Notice the number of valence electrons in the neutral atom, the number of electrons that are lost or gained and the final charge of the ion that is formed.
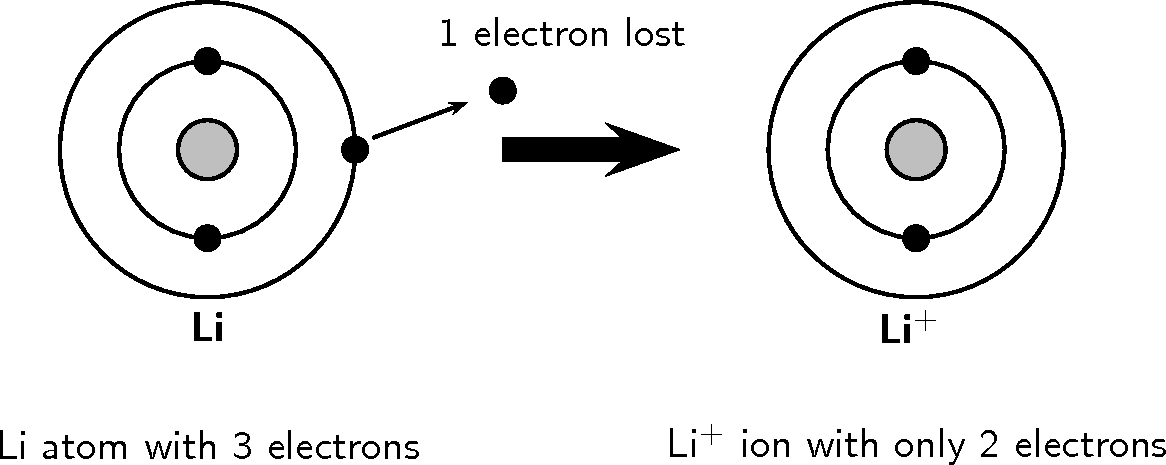
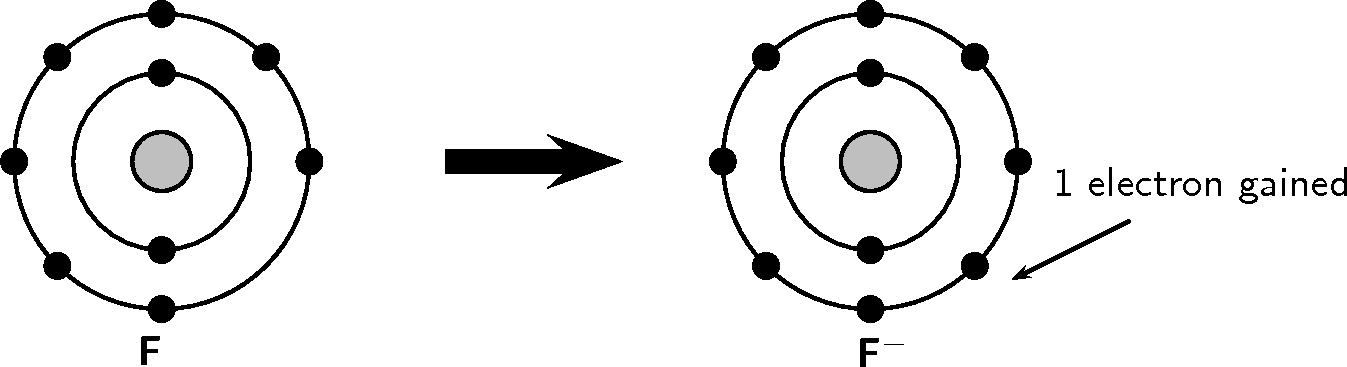
You should have noticed in both these examples that each element lost or gained electrons to make a full outer shell.

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 10 physical science [caps]' conversation and receive update notifications?