| << Chapter < Page | Chapter >> Page > |
Metabolic processes are constantly taking place in the body. Metabolism is the sum of all of the chemical reactions that are involved in catabolism and anabolism. The reactions governing the breakdown of food to obtain energy are called catabolic reactions. Conversely, anabolic reactions use the energy produced by catabolic reactions to synthesize larger molecules from smaller ones, such as when the body forms proteins by stringing together amino acids. Both sets of reactions are critical to maintaining life.
Because catabolic reactions produce energy and anabolic reactions use energy, ideally, energy usage would balance the energy produced. If the net energy change is positive (catabolic reactions release more energy than the anabolic reactions use), then the body stores the excess energy by building fat molecules for long-term storage. On the other hand, if the net energy change is negative (catabolic reactions release less energy than anabolic reactions use), the body uses stored energy to compensate for the deficiency of energy released by catabolism.
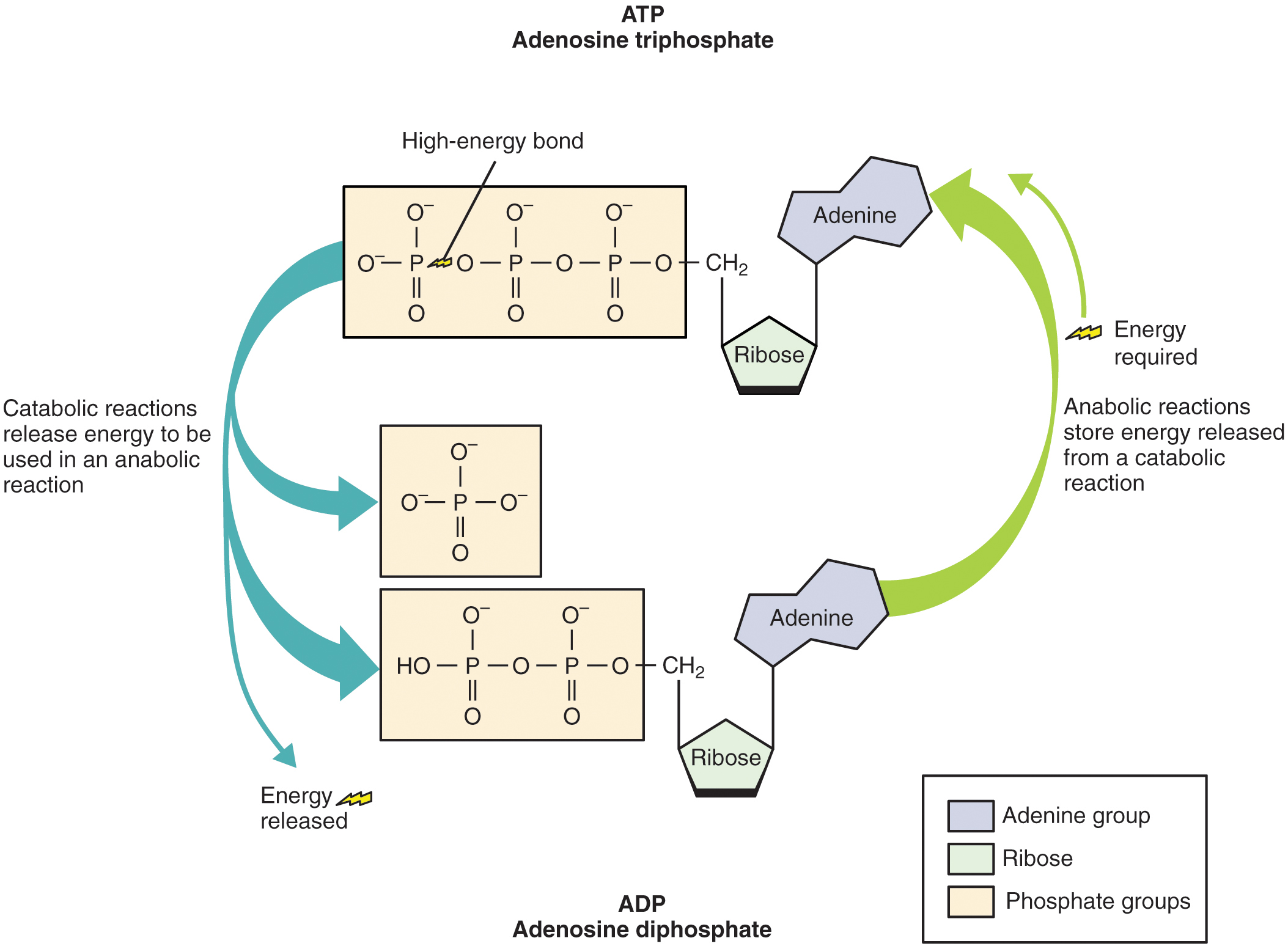
Catabolic reactions break down large organic molecules into smaller molecules, releasing the energy contained in the chemical bonds. These energy releases (conversions) are not 100 percent efficient. The amount of energy released is less than the total amount contained in the molecule. Approximately 40 percent of energy yielded from catabolic reactions is directly transferred to the high-energy molecule adenosine triphosphate (ATP). ATP, the energy currency of cells, can be used immediately to power molecular machines that support cell, tissue, and organ function. This includes building new tissue and repairing damaged tissue. ATP can also be stored to fulfill future energy demands. The remaining 60 percent of the energy released from catabolic reactions is given off as heat, which tissues and body fluids absorb.
Structurally, ATP molecules consist of an adenine, a ribose, and three phosphate groups ( [link] ). The chemical bond between the second and third phosphate groups, termed a high-energy bond, represents the greatest source of energy in a cell. It is the first bond that catabolic enzymes break when cells require energy to do work. The products of this reaction are a molecule of adenosine diphosphate (ADP) and a lone phosphate group (P i ). ATP, ADP, and P i are constantly being cycled through reactions that build ATP and store energy, and reactions that break down ATP and release energy.

Notification Switch
Would you like to follow the 'Anatomy & Physiology: energy, maintenance and environmental exchange' conversation and receive update notifications?