| << Chapter < Page | Chapter >> Page > |
Unlike bryophyte and fern spores (which are haploid cells dependent on moisture for rapid development of gametophytes), seeds contain a diploid embryo that will germinate into a sporophyte. Storage tissue to sustain growth and a protective coat give seeds their superior evolutionary advantage. Several layers of hardened tissue prevent desiccation, and free reproduction from the need for a constant supply of water. Furthermore, seeds remain in a state of dormancy—induced by desiccation and the hormone abscisic acid—until conditions for growth become favorable. Whether blown by the wind, floating on water, or carried away by animals, seeds are scattered in an expanding geographic range, thus avoiding competition with the parent plant.
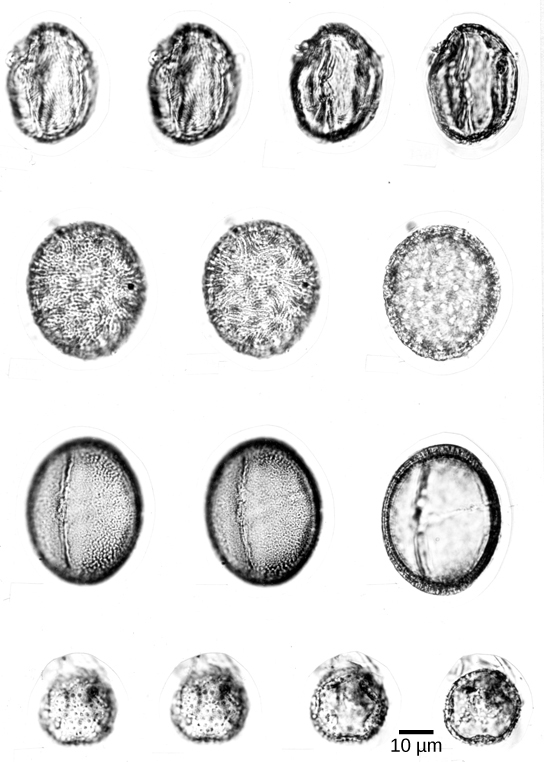
Pollen grains ( [link] ) are male gametophytes and are carried by wind, water, or a pollinator. The whole structure is protected from desiccation and can reach the female organs without dependence on water. Male gametes reach female gametophyte and the egg cell though a pollen tube: an extension of a cell within the pollen grain. The sperm of modern gymnosperms lack flagella, but in cycads and the Gingko , the sperm still possess flagella that allow them to swim down the pollen tube to the female gamete; however, they are enclosed in a pollen grain.
Undisputed fossil records place the massive appearance and diversification of angiosperms in the middle to late Mesozoic era. Angiosperms (“seed in a vessel”) produce a flower containing male and/or female reproductive structures. Fossil evidence ( [link] ) indicates that flowering plants first appeared in the Lower Cretaceous, about 125 million years ago, and were rapidly diversifying by the Middle Cretaceous, about 100 million years ago. Earlier traces of angiosperms are scarce. Fossilized pollen recovered from Jurassic geological material has been attributed to angiosperms. A few early Cretaceous rocks show clear imprints of leaves resembling angiosperm leaves. By the mid-Cretaceous, a staggering number of diverse flowering plants crowd the fossil record. The same geological period is also marked by the appearance of many modern groups of insects, including pollinating insects that played a key role in ecology and the evolution of flowering plants.
Although several hypotheses have been offered to explain this sudden profusion and variety of flowering plants, none have garnered the consensus of paleobotanists (scientists who study ancient plants). New data in comparative genomics and paleobotany have, however, shed some light on the evolution of angiosperms. The two adaptations of flowers and fruit represent an improved reproductive strategy that served to protect the embryo, while increasing genetic variability and range. Angiosperms did not evolve from gymnosperms, but the groups do share a common ancestor ( [link] ).
The most primitive living angiosperm is considered to be Amborella trichopoda , a small plant native to the rainforest of New Caledonia, an island in the South Pacific. Analysis of the genome of A. trichopoda has shown that it is related to all existing flowering plants and belongs to the oldest confirmed branch of the angiosperm family tree. A few other angiosperm groups called basal angiosperms, are viewed as primitive because they branched off early from the phylogenetic tree. Most modern angiosperms are classified as either monocots or eudicots, based on the structure of their leaves and embryos. Basal angiosperms, such as water lilies, are considered more primitive because they share morphological traits with both monocots and eudicots.

Angiosperms produce their gametes in separate organs, which are usually housed in a flower . Both fertilization and embryo development take place inside an anatomical structure that provides a stable system of sexual reproduction largely sheltered from environmental fluctuations. Flowering plants are the most diverse phylum on Earth after insects (Arthropoda); flowers come in a bewildering array of sizes, shapes, colors, smells, and arrangements. Most flowers have a mutualistic pollinator, with the distinctive features of flowers reflecting the nature of the pollination agent. The relationship between pollinator and flower characteristics is one of the great examples of coevolution.
Following fertilization of the egg, the ovule grows into a seed. The surrounding tissues of the ovary thicken, developing into a fruit that will protect the seed and often ensure its dispersal over a wide geographic range. Not all fruits develop from an ovary; such structures are “false fruits.” Like flowers, fruit can vary tremendously in appearance, size, smell, and taste. Tomatoes, walnut shells and avocados are all examples of fruit. As with pollen and seeds, fruits also act as agents of dispersal. Some may be carried away by the wind. Many attract animals that will eat the fruit and pass the seeds through their digestive systems, then deposit the seeds in another location. Cockleburs are covered with stiff, hooked spines that can hook into fur (or clothing) and hitch a ride on an animal for long distances. The cockleburs that clung to the velvet trousers of an enterprising Swiss hiker, George de Mestral, inspired his invention of the loop and hook fastener he named Velcro.

Notification Switch
Would you like to follow the 'Principles of biology' conversation and receive update notifications?