| << Chapter < Page | Chapter >> Page > |
In chemiosmosis, the free energy from the series of redox reactions just described is used to pump hydrogen ions (protons) across the membrane (from inside the matrix into the intermembrane space). The uneven distribution of H + ions across the membrane establishes both concentration and electrical gradients (thus, an electrochemical gradient), sue to the hydrogen ions’ positive charge and their aggregation on one side of the membrane. This electrochemical gradient is a form of potential energy, and its discovery was a key step in Mitchell's elucidation of the details of oxidative phosphorylation.
If the membrane was not a barrier to the movement of the hydrogen ions, the ions would diffuse back across into the matrix, driven by their electrochemical gradient. But recall that many ions cannot diffuse through the nonpolar regions of phospholipid membranes without the aid of ion channels. Similarly, hydrogen ions in the intermembrane space can only pass through the inner mitochondrial membrane through an integral membrane protein called ATP synthase ( [link] ). This complex protein acts as a tiny generator, turned by the force of the hydrogen ions diffusing down their electrochemical gradient via a channel in the protein. This powers an actual rotation of parts of the enzyme; the rotation of parts of this molecular machine facilitates the addition of a phosphate to ADP, forming ATP. In the absence of a hydrogen ion gradient, the rotation stops and no ATP is made.
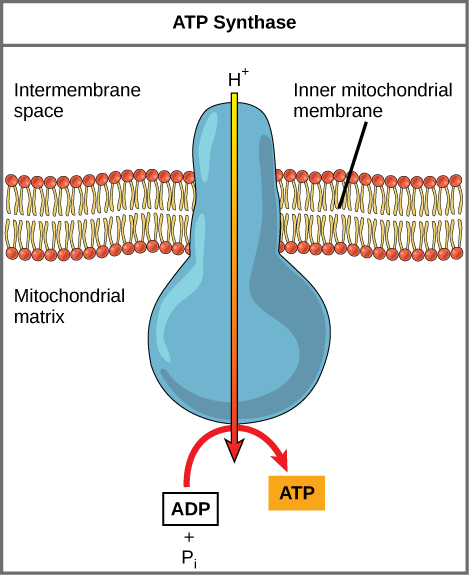
Chemiosmosis ( [link] ) is used to generate 90 percent of the ATP made during aerobic cellular respiration; it is also the method used in the light reactions of photosynthesis to harness the energy of sunlight in the process of photophosphorylation. Recall that the production of ATP using the process of chemiosmosis in mitochondria is called oxidative phosphorylation. The overall result of these reactions is the production of about 32 ATP from the energy of the electrons removed from hydrogen atoms ( [link] ). These electrons were originally part of a glucose molecule. At the end of the pathway, the electrons are used to reduce an oxygen molecule to oxygen ions. The extra electrons on the oxygen attract hydrogen ions (protons) from the surrounding medium, and water is formed.
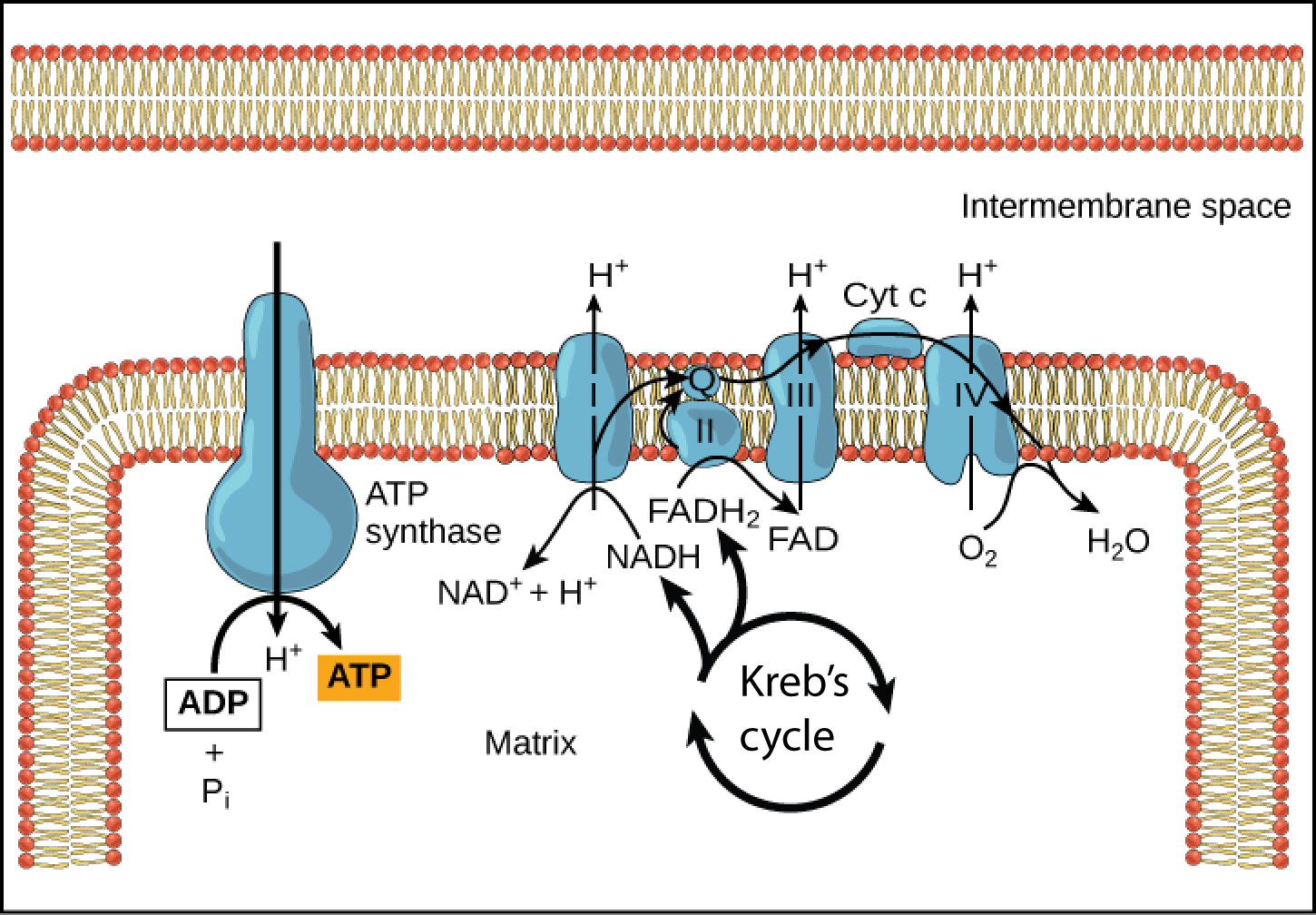
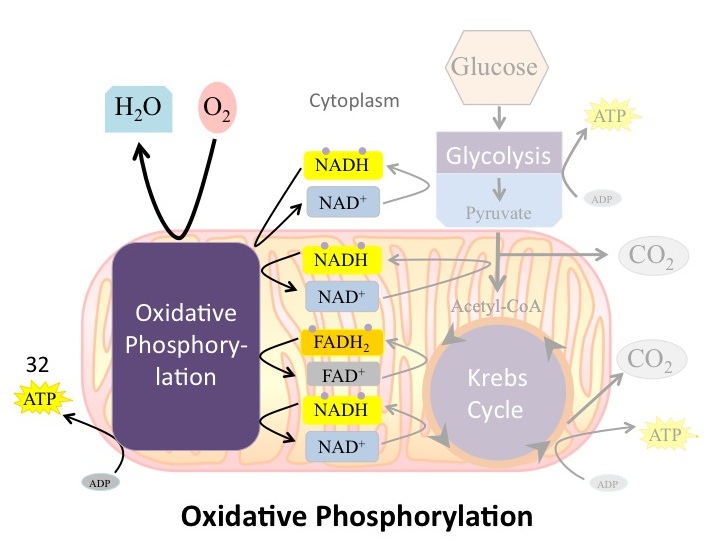
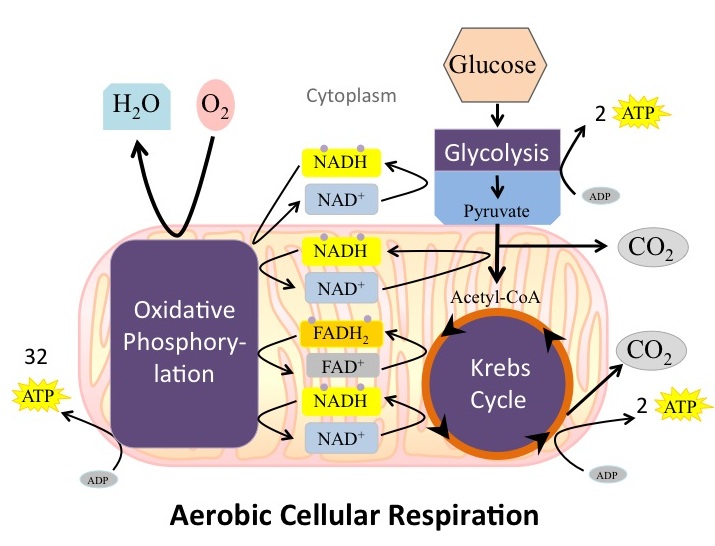
The number of ATP molecules generated from the catabolism of glucose can be variable. For example, the number of hydrogen ions that the electron transport chain complexes can pump through the membrane varies between species. Another source of variance stems from the shuttle of electrons across the membranes of the mitochondria. (The NADH generated from glycolysis cannot easily enter mitochondria.) Thus, electrons are picked up on the inside of mitochondria by either NAD
+ or FAD
+ . As you have learned earlier, these FAD
+ molecules carry electrons that are lower in energy than those on NADH; consequently, fewer ATP molecules are generated when FAD
+ acts as a carrier. NAD
+ is used as the electron transporter in the liver and FAD
+ acts in the brain. Assuming optimal ATP production, a net of 2 are formed in glycolysis, 2 are formed in the Kreb's cycle, and 32 produced during oxidative phophorylation (
[link] ). The total amount of ATP formed form 1 molecule of glucose is generally around 36 under optimal conditions.

Notification Switch
Would you like to follow the 'Principles of biology' conversation and receive update notifications?