| << Chapter < Page | Chapter >> Page > |
Electrostatics is the study of electric charge which is static (not moving). In this chapter we will look at some of the basic principle of electric charge as well as the principle of conservation of charge.
All objects surrounding us (including people!) contain large amounts of electric charge. There are two types of electric charge: positive charge and negative charge. If the same amountsof negative and positive charge are brought together, they neutralise each other and there is no net charge . Neutral objects are objects which contain equal amouts of positive and negative charges. However, if there is a little bit more of one type of charge than the other on theobject then the object is said to be electrically charged . The picture below shows what the distribution of charges might look like for a neutral, positively charged andnegatively charged object.
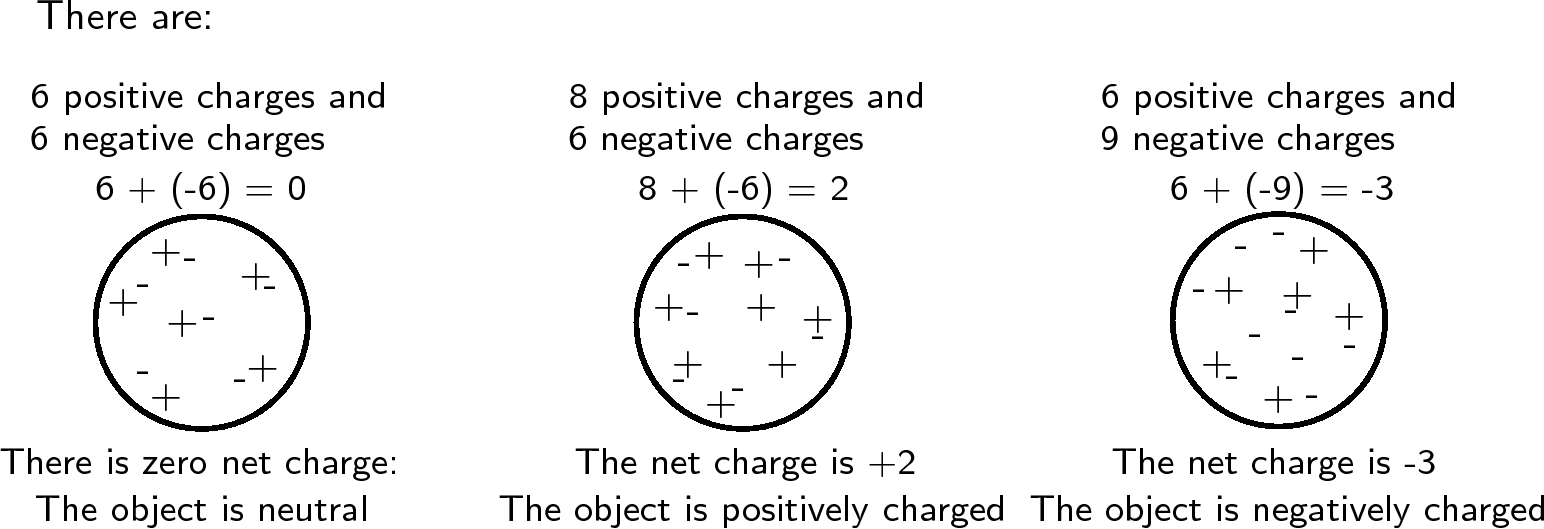
Charge is measured in units called coulombs (C) . A coulomb of charge is a very large charge. In electrostatics we therefore often work with charge in microcoulombs ( ) and nanocoulombs ( ).
Objects may become charged in many ways, including by contact with or being rubbed by other objects. This means that they can gain extra negative or positive charge. For example, charging happens when you rub your feet against the carpet. When youthen touch something metallic or another person, you feel a shock as the excess charge that you have collected is discharged .
The principle of conservation of charge states that the net charge of an isolated system remains constant during any physical process, e.g. when two charges make contact and are separated again.
When you rub your feet against the carpet, negative charge is transferred to youfrom the carpet. The carpet will then become positively charged by the same amount .
Another example is to take two neutral objects such as a plastic ruler and a cotton cloth (handkerchief). To begin, the two objects are neutral (i.e. have the same amounts of positive and negative charge).
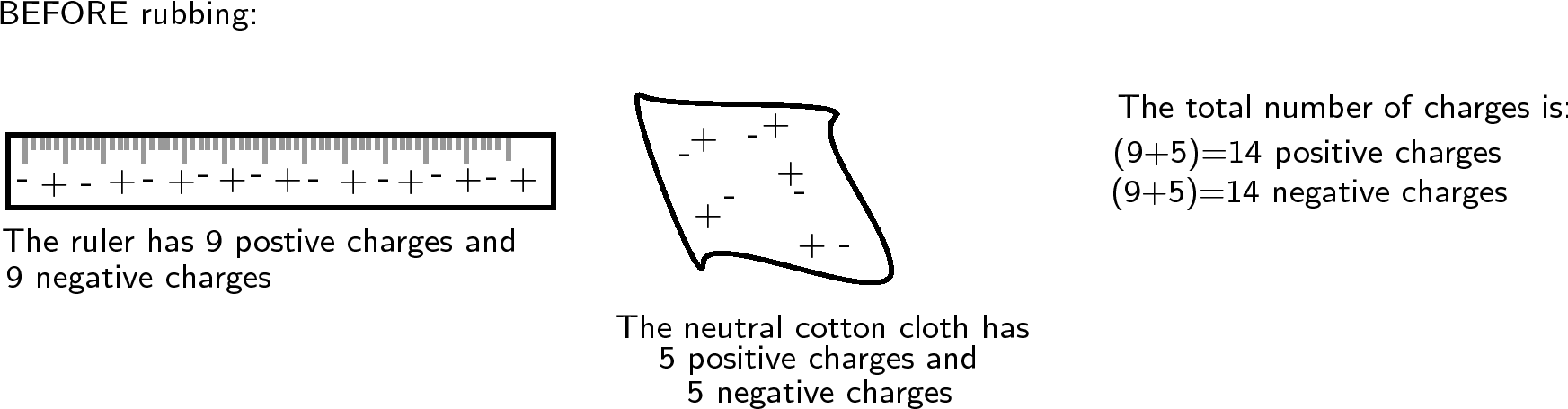
Now, if the cotton cloth is used to rub the ruler, negative charge is transferred from the cloth to the ruler. The ruler is now negatively charged (i.e. has an excess of electrons) and the cloth is positively charged (i.e. is electron deficient). If you count up all the positive and negative charges at the beginning and the end, there are still the same amount. i.e. total charge has been conserved !
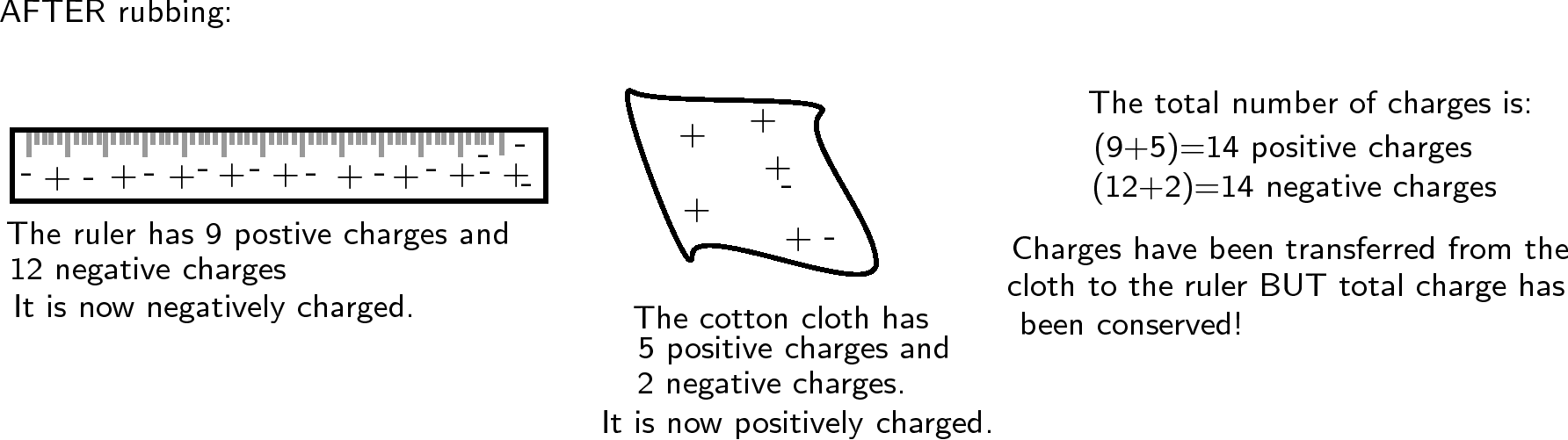
Note that in this example the numbers are made up to be easy to calculate. In the real world only a tiny fraction of the charges would move from one object to the other, but the total charge would still be conserved.
The following simulation will help you understand what happens when you rub an object against another object.
run demo

Notification Switch
Would you like to follow the 'Physics - grade 10 [caps 2011]' conversation and receive update notifications?