| << Chapter < Page | Chapter >> Page > |
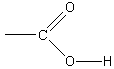
We consider first the simple binary acids. HCl,HBr, andHI are all strong acids, whereasHF is a weak acid. In comparing the experimental values of values in [link] , we note that the acid strength increases in the orderHF<HCl<HBr<HI. This means that the hydrogen ion can more readily separate from thecovalent bond with the halogen atom (X) as we move down the periodic table. This is reasonable, because the strength of the H-Xbond also decreases as we move down the periodic table, as shown in [link] .
| pK a | Bond Energy ( ) | |
|---|---|---|
| HF | 3.1 | 567.7 |
| HCl | -6.0 | 431.6 |
| HBr | -9.0 | 365.9 |
| HI | -9.5 | 298.0 |
The decreasing strength of the H-X bond is primarily due to the increase is the size of the X atom as we movedown the periodic table. We conclude that one factor which influences acidity is the strength of the H-X bond: a weaker bondproduces a stronger acid, and vice versa.
In the acids in the other two categories, the hydrogen atom which ionizes is attached directly to an oxygen atom.Thus, to understand acidity in these molecules, we must examine what the oxygen atom is in turn bonded to. It is very interestingto note that, in examining compounds like R-O-H, where R is an atom or group of atoms, we can get either acidic or basic properties.For examples, NaOHis a strong base, whereas HOClis a weak acid. This means that, when NaOHionizes in solution, the Na-O linkage ionizes, whereas when HOClionizes in solution, the H-O bond ionizes.
To understand this behavior, we compare the strength of the simple oxyacidsHOI, HOBr,and HOCl.The 's for these acids are found experimentally to be, respectively, 10.6,8.6, and 7.5. The acid strength for HOXincreases as we move up the periodic table in the halogen group. This means that the H-O bond ionizes more readily when the oxygenatom is bonded to a more electronegative atom.
We can add to this observation by comparing
the strengths of the acidsHOCl,
HOClO,HOClO
2 ,
andHOClO
3 .
(Note that the molecular formulae are more commonly written asHClO,
HClO
2 ,
HClO
3 ,
andHClO
4 .
We have written them instead to emphasize the molecular structure.)The
's
of these acids are, respectively, 7.5, 2.0, -2.7, and -8.0.In each case, the molecule with more oxygen atoms on the central Cl
atom is the stronger acid:HOClO
is more acidic thanHOCL,
Why would electronegativity play a role in acid strength? There are two conclusions we might draw. First, agreater electronegativity of the atom or atoms attached to the H-O in the oxyacid apparently results in a weaker H-O bond, which isthus more readily ionized. We know that an electronegative atom polarizes bonds by drawing the electrons in the molecule towardsit. In this case, the Cl in HOCland the Br in HOBrmust polarize the H-O bond, weakening it and facilitating the ionization of the hydrogen. In comparingHOCl toHOClO, the added oxygen atom must increase the polarization of the H-Obond, thus weakening the bond further and increasing the extent of ionization.

Notification Switch
Would you like to follow the 'Ucd bis2a intro to biology v1.2' conversation and receive update notifications?