| << Chapter < Page | Chapter >> Page > |
In the oil industry, Portland cement supports boreholes of ever increasing depth. This application requires a high degree of control over the setting kinetics to allow the cement to be pumped down in a liquid form. A number of chemical inhibitors are employed to delay the setting time. The ideal inhibitor for oil well cementing would predictably delay the setting of cement, and then suddenly allow hydration to continue at a rapid rate.
A wide range of compounds show set inhibition of the hydration of Portland cement. Some common examples include, sucrose, tartaric acid, gluconic acid δ-lactone, lignosulfonate, and organic phosphonic acids, in particular nitrilo-tris(methylene)phosphonic acid (H 6 ntmp). The structures of these retarders are shown in [link] .
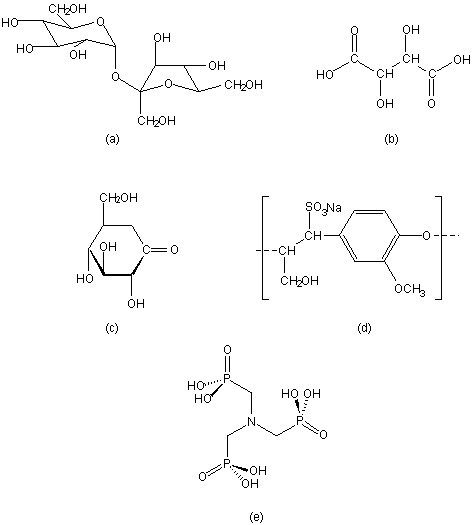
In spite of the fact that the science of cement hydration inhibition has been investigated for over 40 years, the mechanistic details are still the subject of much speculation. There are five primary models for cement hydration inhibition: calcium complexation, nucleation poisoning, surface adsorption, protective coating/osmotic bursting, and dissolution-precipitation. A summary of the characteristic behavior of selected retarders is shown in [link] .
| Retarder | Characteristic behavior |
| sucrose | Ca binding, acts directly on silicates, accelerates ettringite formation |
| tartaric acid | acts via calcium complexation and calcium tartrate coating, inhibits ettringite formation |
| lignosulfonate | accelerates ettringite formation, calcium becomes incorporated into the polymer matrix during hydration, forms a diffusion barrier |
| nitrilo-tris(methylene)phosphonic acid (H 6 ntmp) | promotes Ca dissolution, forms [Ca(H 6 ntmp)], heterogeneous nucleation on aluminates creates a protective coating around the grain |
Inhibition by calcium complexation relies largely on the requirement that small calcium oxide/hydroxide templates must form in the pore water of cement pastes before silicate tetrahedra can condense into dimeric and oligomeric silicates to form C–S–H. Calcium complexation involves either removing calcium from solution by forming insoluble salts, or chelating calcium in solution. Calcium complexation lowers the amount of calcium effectively in solution, delaying the time to Ca(OH) 2 super-saturation and preventing precipitation of the necessary templates. Simple calcium complexation should dramatically increase the amount of Si(OH) 4 tetrahedra in solution, and indeed this is observed with most retarders. However, if the retarder were acting solely by calcium complexation, then one molecule of retarder would be required per calcium ion in solution, and good inhibitors are used in much smaller quantities, on the order of 0.1-2% by weight of cement. In addition, there is no simple correlation between either calcium binding strength or calcium salt solubility and retarding ability. Yet it has been shown that in pure systems, i.e., of C3S and C2S, that the lime concentration in solutions is the most important factor in determining the precipitation of C–S–H. Therefore, although calcium complexation must play some role in inhibition, other mechanisms of inhibition must be at work as well. An example of a retarder that operates primarily through calcium complexation is tartaric acid, however, the formation of insoluble calcium tartrate on cement grains suggest that dissolution/precipitation occurs in addition ( [link] ).

Notification Switch
Would you like to follow the 'Portland cement in the energy industry' conversation and receive update notifications?