| << Chapter < Page | Chapter >> Page > |
We noted in the introduction that atoms in elemental form are neutral, or without charge. Since atoms contain negatively charged electrons, each atom must also contain a positive charge which exactly equals the total charge on the electrons in the atom. Since electrons are particles, there has to be an integer number of negative charges, so there also has to be an integer number of positive charges in each atom. These statements are important to keep in mind, but they don’t help us very much without more data. We don’t know how many electrons are in each atom, and therefore we don’t know how many positive charges are in each atom. We do not even know whether that number of electrons is the same or different for different atoms of different elements.
In addition, we don’t know how these positive and negative charges are arranged in each atom. They might be clustered together in some sort of ball, they might be paired off together, they might be randomly arranged, or there may be some other arrangement that we don’t expect.
In this concept development study, we will determine the arrangement of the charged particles in an atom, and we will determine the numbers of charged particles in the atoms of each element.
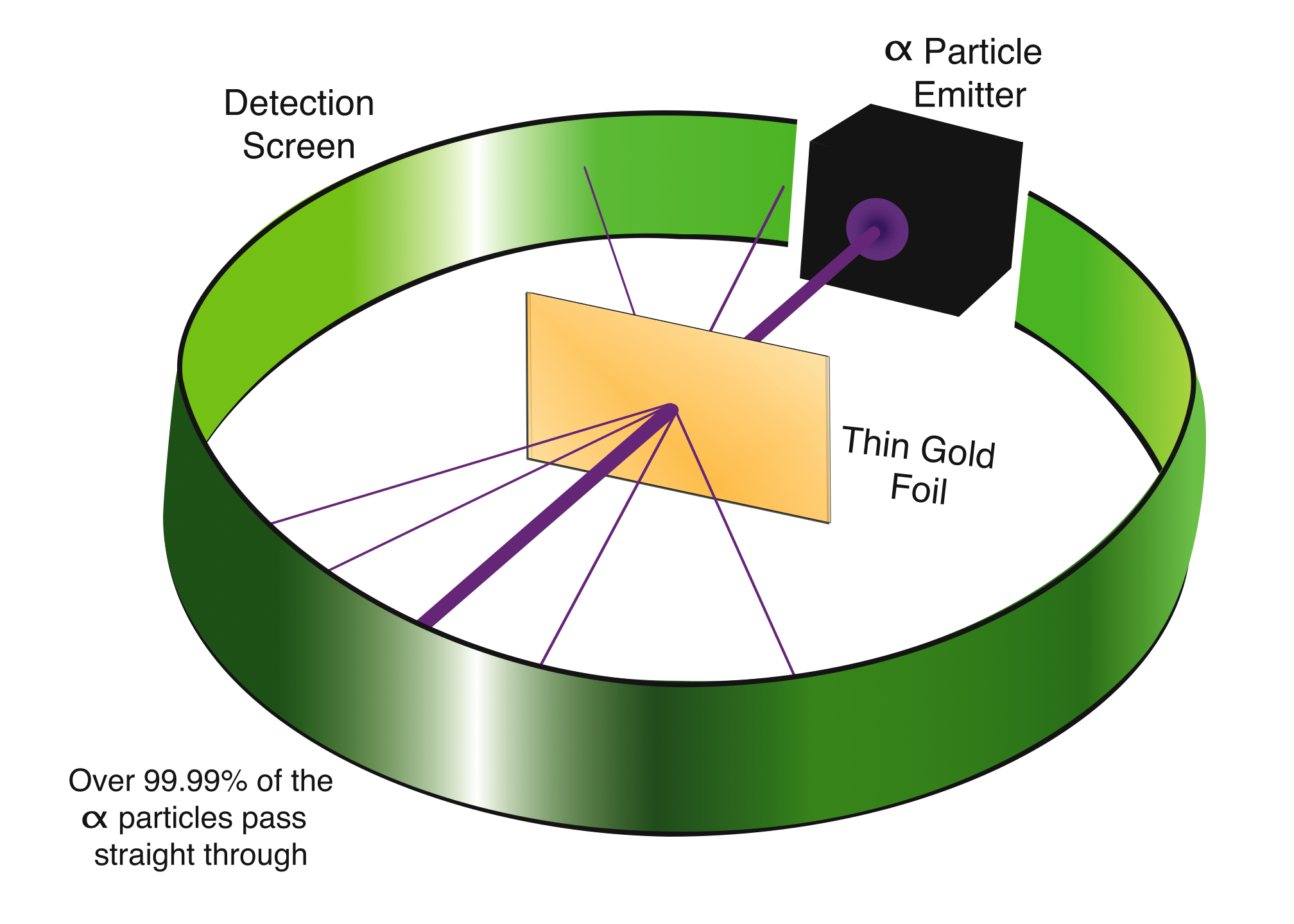
To find out what the inside of an atom looks like, we perform a “scattering” experiment. This involves shooting charged particles at atoms and watching what happens. Depending on how the charged particles are arranged inside the atom, these charges should “scatter” the particles we shoot. If we look at the pattern of the scattering, we should be able to infer what the arrangement of the charges inside the atom looks like.
It would seem very hard to shoot particles at individual atoms, though, since these are very small, impossible to see, and possibly even elusive. One way to do this is to take a very thin sheet of a metal, so thin that the thickness of the metal will not be very many atoms across. Gold is a good choice of metal, since it is easy to hammer out to a very thin sheet and it is very unreactive, so it is possible to make it quite pure. In this experiment, the thickness of the gold foil will be only about 10 -4 cm, sometimes called 1 “micron.” This is less than one-twentieth of the thickness of one human hair, so this is very thin indeed.
We need particles to shoot at this thin foil, preferably charged particles which will interact with the positive and negative charges in the gold atoms. A good choice is the α particle, which is positively charged and much more massive than an electron. In the experiment, we will fire a beam of α particles directly at the gold foil, and then we will observe where the α particles go after interacting with the gold atoms. They might pass through the foil, they might be deflected somewhat as they pass through, or they might even rebound in various angles back to the source of the beam.

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2012' conversation and receive update notifications?