| << Chapter < Page | Chapter >> Page > |
This reasoning reveals that the amounts of
reactant and product present at equilibrium are determined by therates of the forward and reverse reactions. If the rate of the
forward reaction (
It was noted above that the equilibriumpartial pressures of the gases in a reaction vary depending upon a variety of conditions. These include changes in the initial numbersof moles of reactants and products, changes in the volume of the reaction flask, and changes in the temperature. We now study thesevariations quantitatively.
Consider first the reaction here . Following on our previous study of this reaction, we inject an initial amount ofN 2 O 4 (g) into a 100L reaction flask at 298K. Now, however, we vary theinitial number of moles of N 2 O 4 (g) in the flask and measure the equilibrium pressures of both thereactant and product gases. The results of a number of such studies are given here .
| Initial | (atm) | (atm) |
|---|---|---|
| 0.1 | 0.00764 | 0.033627 |
| 0.5 | 0.071011 | 0.102517 |
| 1 | 0.166136 | 0.156806 |
| 1.5 | 0.26735 | 0.198917 |
| 2 | 0.371791 | 0.234574 |
| 2.5 | 0.478315 | 0.266065 |
| 3 | 0.586327 | 0.294578 |
| 3.5 | 0.695472 | 0.320827 |
| 4 | 0.805517 | 0.345277 |
| 4.5 | 0.916297 | 0.368255 |
| 5 | 1.027695 | 0.389998 |
We might have expected that the amount of NO 2 produced at equilibrium would increase in direct proportion to increases in the amount ofN 2 O 4 we begin with. [link] shows that this is not the case. Note that when we increase the initial amountof N 2 O 4 by a factor of 10 from 0.5 moles to 5.0 moles, the pressure of NO 2 at equilibrium increases by a factor of less than 4.
The relationship between the pressures at equilibrium and the initial amount ofN 2 O 4 is perhaps more easily seen in a graph of the data in [link] , as shown in [link] . There are some interesting features here. Note that, when the initial amount ofN 2 O 4 is less than 1 mol, the equilibrium pressure of NO 2 is greater than that of N 2 O 4 . These relative pressures reverse as the initial amount increases,as the N 2 O 4 equilibrium pressure keeps track with the initial amount but the NO 2 pressure falls short. Clearly, the equilibrium pressure of NO 2 does not increase proportionally with the initial amount of N 2 O 4 . In fact, the increase is slower than proportionality, suggestingperhaps a square root relationship between the pressure of NO 2 and the initial amount of N 2 O 4 .
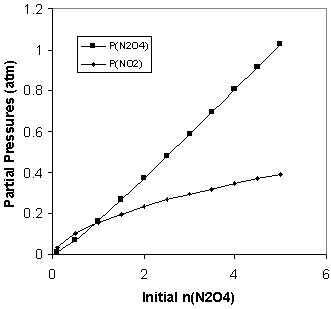
We test this in [link] by plotting at equilibrium versus the square root of the initial number of moles ofN 2 O 4 . [link] makes it clear that this is not a simple proportional relationship, but it is closer. Note in [link] that the equilibrium pressure increases close to proportionally with the initial amount of N 2 O 4 . This suggests plotting versus the square root of . This is done in [link] , where we discover that there is a very simple proportional relationshipbetween the variables plotted in this way. We have thus observed that

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2012' conversation and receive update notifications?