| << Chapter < Page | Chapter >> Page > |
Various morphologies of numerous oxide nanocrystals including Fe, Co, Mn ferrites, Co 3 O 4 , Cr 2 O 3 , MnO, NiO, ZnO, and others have been obtained by pyrolysis of metal carboxylates in the presence of different fatty acids (oleic, myristic). Control over the chemical composition of the nanoparticle is readily attained through the relative concentration of reagents used for nanoparticle growth. In many systems there is a direct linear relationship between the relative composition in the nano particles and the reagent solutions used.
As described above the "bottom-up" approach of reacting small inorganic molecules to form oligomeric and polymeric materials and subsequently nano particles is a common approach for a wide range of metal and non-metal oxides. However, in the case of aluminum oxide nanoparticles, the relative rate of the hydrolysis and condensation reactions often makes particle size control difficult. Once the structure of alumina sol-gels (known as alumoxanes) had been determined to comprise of a boehmite-like nanoparticle core, it was proposed that alumina nanoparticles could be prepared directly from the mineral. Such a "top-down" approach represented a departure from the traditional synthetic methodologies. Thus, it has been shown that carboxylic acids (RCO 2 H) react with boehmite, [Al(O)(OH)] n , to yield the appropriate carboxy-alumoxane.
[Al(O)(OH)] n + HO 2 CR → [Al(O) x (OH) y (O 2 CR) z ] n
Initial syntheses were carried out using the acid as the solvent or xylene, however, subsequent research demonstrated the use of water as a solvent and acetic acid as the most convenient capping agent. A solventless synthesis has also been developed. Thus, the synthesis of alumoxane nanoparticles may be summarized as involving the reaction between dirt (boehmite), vinegar (acetic acid), and water. The function of the acid is two-fold. First, to cleave the mineral lattice and “carve out” nanoscale fragment, and second to provide a chemical cap to the fragment ( [link] ).
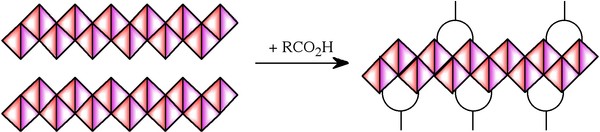
The carboxylate-alumoxane nanoparticles prepared from the reaction of boehmite and carboxylic acids are air and water stable. The soluble carboxylate-alumoxanes can be dip-coated, spin coated, and spray-coated onto various substrates. The size of the alumoxane nanoparticles is dependant on the substituents, the reaction conditions (concentration, temperature, time, etc.), and the pH of the reaction solution. Unlike other forms of oxide nanoparticle, the alumoxanes are not mono-dispersed but have a range of particle sizes. Also unlike other metal oxide nanoparticles, the core of the alumoxane can undergo a low temperature reaction that allows for the incorporation of other metals (e.g., Ti, La, Mo, V, Ca). This occurs by reaction of metal acetylacetenoates [M(acac) n ] with the carboxylate alumoxane ( [link] ).
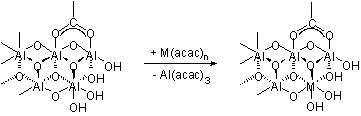
Given the analogous structure of Fe(O)(OH) (lepidocrocite) to boehmite, it is not surprising that the iron analog of alumoxane nanoparticles (i.e., ferroxanes) is readily prepared. Ferroxanes have been extensively characterized, and have shown to have identical structural features to alumoxanes and undergo similar exchange reactions.

Notification Switch
Would you like to follow the 'Nanomaterials and nanotechnology' conversation and receive update notifications?