| << Chapter < Page | Chapter >> Page > |
At its most fundamental level, life is made up of matter. Matter occupies space and has mass. All matter is composed of elements , substances that cannot be broken down or transformed chemically into other substances. Each element is made of one type of atoms.
Each element is designated by its chemical symbol (such as H, N, O, C, and Na), and possesses unique properties. These unique properties allow elements to combine and to bond with each other in specific ways.
An atom is the smallest component of an element that retains all of the chemical properties of that element. For example, one hydrogen atom has all of the properties of the element hydrogen, such as it exists as a gas at room temperature, and it bonds with oxygen to create a water molecule. Hydrogen atoms cannot be broken down into anything smaller while still retaining the properties of hydrogen. If a hydrogen atom were broken down into subatomic particles, it would no longer have the properties of hydrogen.
At the most basic level, all organisms are made of a combination of elements. They contain atoms that combine together to form molecules. In multicellular organisms, such as animals, molecules can interact to form cells that combine to form tissues, which make up organs. These combinations continue until entire multicellular organisms are formed.
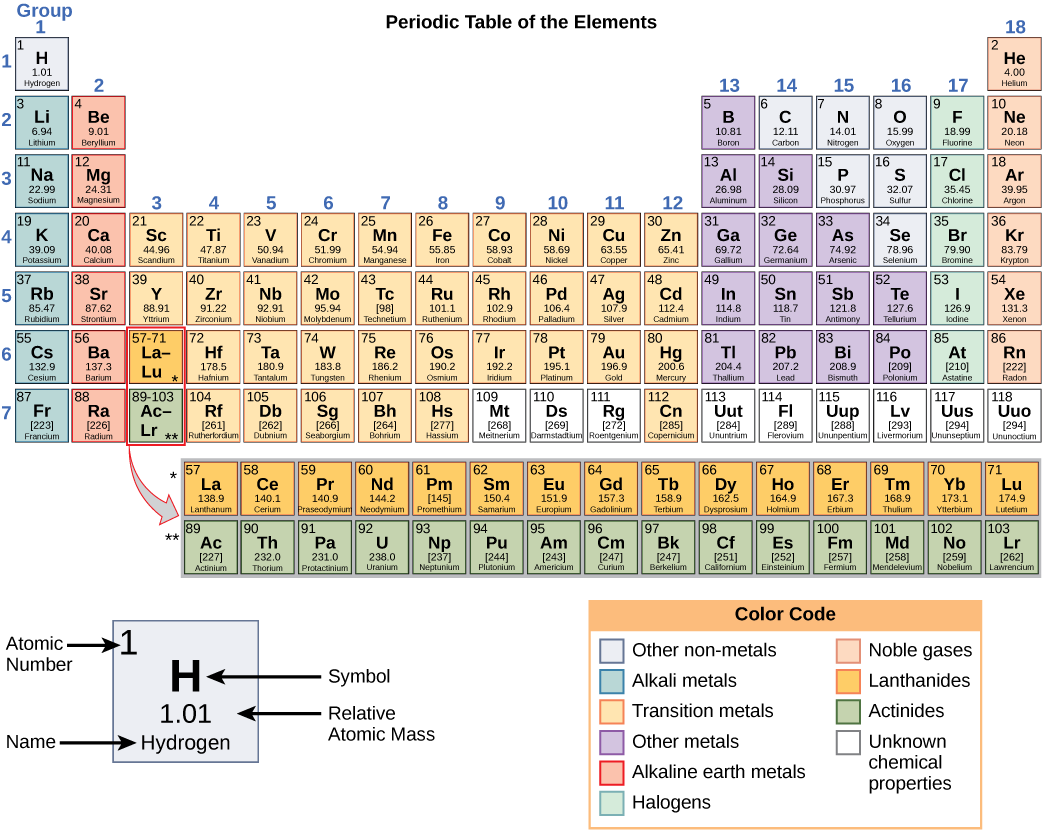
There are two types of bonds that hold atoms together; polar and non-polar. Non-polar bonds form non-polar molecules with no charge on them, like carbon with carbon or carbon with hydrogen. Polar bonds form polar molecules with a partial charge, either positive or negative.
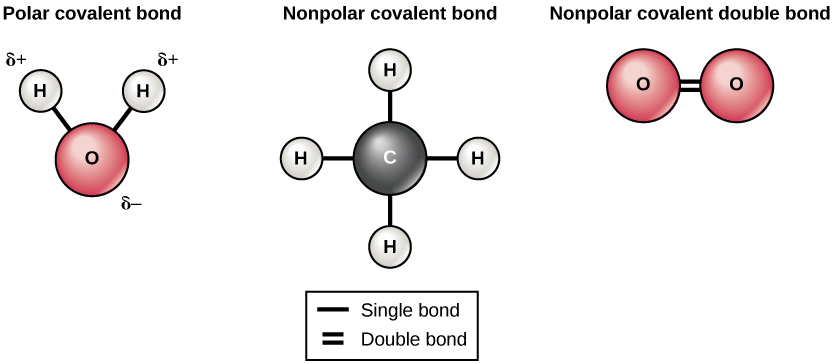
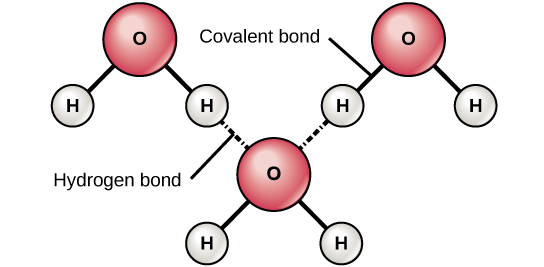
Hydrogen bonds can form between different molecules and they do not always have to include a water molecule. Hydrogen atoms in polar bonds within any molecule can form bonds with other adjacent molecules. For example, hydrogen bonds hold together two long strands of DNA to give the DNA molecule its characteristic double-stranded structure. Hydrogen bonds are also responsible for some of the three-dimensional structure of proteins.
Matter is anything that occupies space and has mass. It is made up of atoms of different elements. All of the 92 elements that occur naturally have unique qualities that allow them to combine in various ways to create compounds or molecules. Atoms, which consist of protons, neutrons, and electrons, are the smallest units of an element that retain all of the properties of that element.

Notification Switch
Would you like to follow the 'Environmental biology' conversation and receive update notifications?