| << Chapter < Page | Chapter >> Page > |
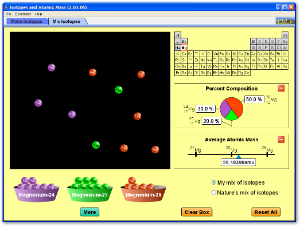
This simulation allows you to see how isotopes and relative atomic mass are inter related.
| Isotope | Z | A | Protons | Neutrons | Electrons |
| Carbon-12 | |||||
| Carbon-14 | |||||
| Chlorine-35 | |||||
| Chlorine-37 |
Standard notation shows the chemical symbol, the atomic mass number and the atomic number of an element as follows:
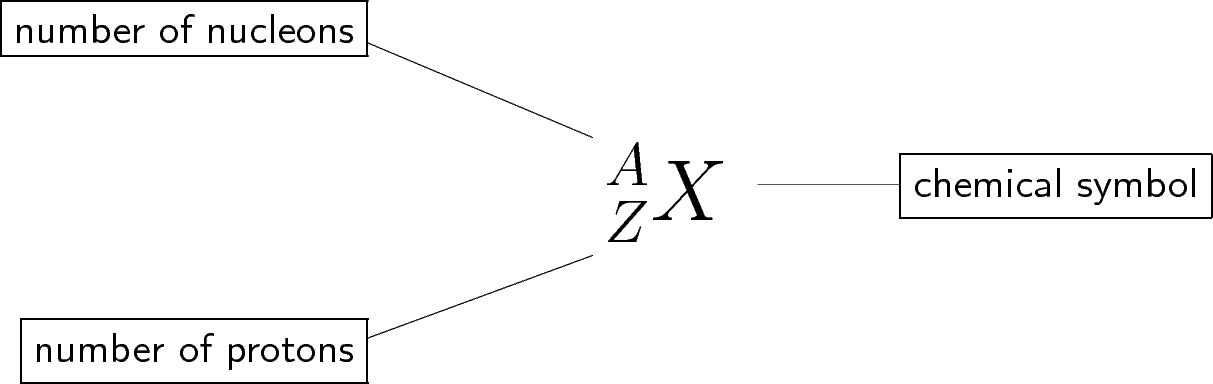
For example, the iron nucleus which has 26 protons and 30 neutrons, is denoted as:
where the atomic number is
The following worked examples will help you to understand the concept of an isotope better.
For the element (uranium), use standard notation to describe:
We know that isotopes of any element have the same number of protons (same atomic number)in each atom, which means that they have the same chemical symbol. However, they have a different number of neutrons, and therefore a different mass number.
Therefore, any isotope of uranium will have the symbol:
Also, since the number of protons in uranium isotopes is always the same, we can write down the atomic number:
Now, if the isotope we want has 2 fewer neutrons than , then we take the original mass number and subtract 2, which gives:
Following the steps above, we can write the isotope with 4 more neutrons as:
Which of the following are isotopes of ?
We know that isotopes have the same atomic number but different mass numbers.
You need to look for the element that has the same atomic number but a different atomic mass number. The only element is . What is different is that there are 2 more neutrons than in the original element.
For the sulphur isotope , give the number of...
, therefore the number of protons is 16 (answer to (a)).
, therefore the number of nucleons is 33 (answer to (b)).
The atom is neutral, and therefore the number of electrons is the same as the number of protons. The number of electrons is 16 (answer to (c)).
The number of neutrons is 17 (answer to (d)).
It is important to realise that the atomic mass of isotopes of the same element will be different because they have a different number of nucleons. Chlorine, for example, has two common isotopes which are chlorine-35 and chlorine-37. Chlorine-35 has an atomic mass of 35 u, while chlorine-37 has an atomic mass of 37 u. In the world around us, both of these isotopes occur naturally. It doesn't make sense to say that the element chlorine has an atomic mass of 35 u, or that it has an atomic mass of 37 u. Neither of these are absolutely true since the mass varies depending on the form in which the element occurs. We need to look at how much more common one is than the other in order to calculate the relative atomic mass for the element chlorine. This is the number that you find on the Periodic Table.
The relative atomic mass of some elements depends on where on Earth the element is found. This is because the isotopes can be found in varying ratios depending on certain factors such as geological composition, etc. The International Union of Pure and Applied Chemistry (IUPAC) has decided to give the relative atomic mass of some elements as a range to better represent the varying isotope ratios on the Earth. For the calculations that you will do at high school, it is enough to simply use one number without worrying about these ranges.
The element chlorine has two isotopes, chlorine-35 and chlorine-37. The abundance of these isotopes when they occur naturally is 75% chlorine-35 and 25% chlorine-37. Calculate the average relative atomic mass for chlorine.
Contribution of
Contribution of
If you look on the periodic table, the average relative atomic mass for chlorine is . You will notice that for many elements, the relative atomic mass that is shown is not a whole number. You should now understand that this number is the average relative atomic mass for those elements that have naturally occurring isotopes.
This simulation allows you to see how isotopes and relative atomic mass are inter related.

| Isotope | Z | A | Protons | Neutrons | Electrons |
| Carbon-12 | |||||
| Carbon-14 | |||||
| Chlorine-35 | |||||
| Chlorine-37 |

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 10 physical science [caps]' conversation and receive update notifications?