| << Chapter < Page | Chapter >> Page > |
Airbags ( [link] ) are a safety feature provided in most automobiles since the 1990s. The effective operation of an airbag requires that it be rapidly inflated with an appropriate amount (volume) of gas when the vehicle is involved in a collision. This requirement is satisfied in many automotive airbag systems through use of explosive chemical reactions, one common choice being the decomposition of sodium azide, NaN 3 . When sensors in the vehicle detect a collision, an electrical current is passed through a carefully measured amount of NaN 3 to initiate its decomposition:
This reaction is very rapid, generating gaseous nitrogen that can deploy and fully inflate a typical airbag in a fraction of a second (~0.03–0.1 s). Among many engineering considerations, the amount of sodium azide used must be appropriate for generating enough nitrogen gas to fully inflate the air bag and ensure its proper function. For example, a small mass (~100 g) of NaN 3 will generate approximately 50 L of N 2 .

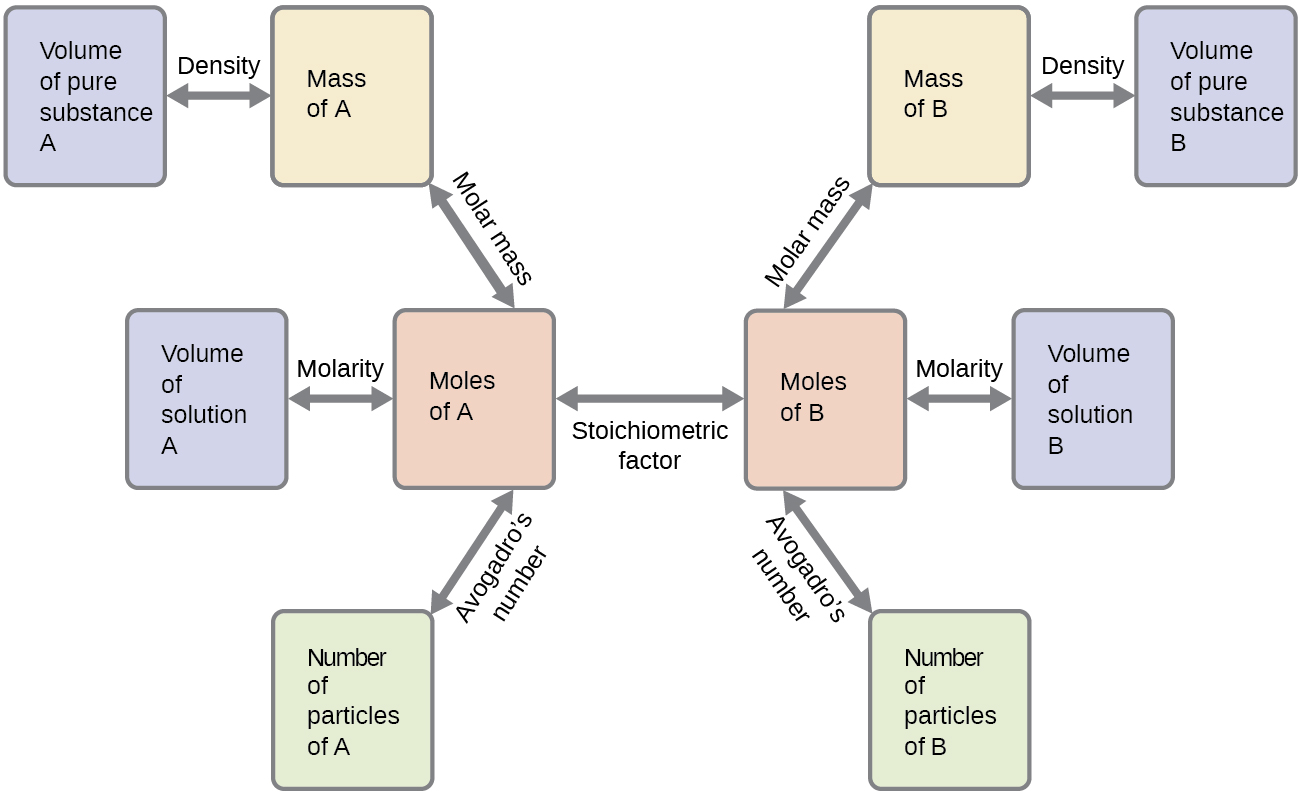
A balanced chemical equation may be used to describe a reaction’s stoichiometry (the relationships between amounts of reactants and products). Coefficients from the equation are used to derive stoichiometric factors that subsequently may be used for computations relating reactant and product masses, molar amounts, and other quantitative properties.
Write the balanced equation, then outline the steps necessary to determine the information requested in each of the following:
(a) The number of moles and the mass of chlorine, Cl 2 , required to react with 10.0 g of sodium metal, Na, to produce sodium chloride, NaCl.
(b) The number of moles and the mass of oxygen formed by the decomposition of 1.252 g of mercury(II) oxide.
(c) The number of moles and the mass of sodium nitrate, NaNO 3 , required to produce 128 g of oxygen. (NaNO 2 is the other product.)
(d) The number of moles and the mass of carbon dioxide formed by the combustion of 20.0 kg of carbon in an excess of oxygen.
(e) The number of moles and the mass of copper(II) carbonate needed to produce 1.500 kg of copper(II) oxide. (CO 2 is the other product.)
(f)
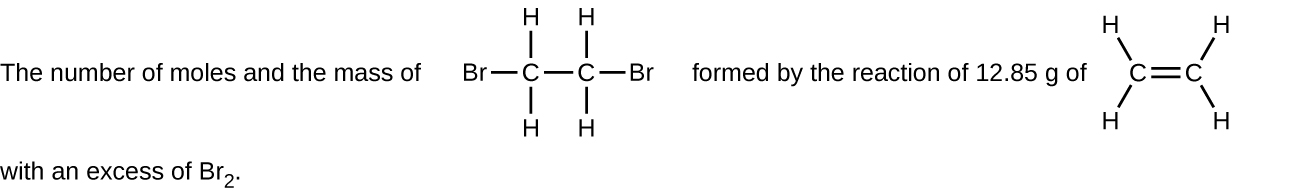
Determine the number of moles and the mass requested for each reaction in [link] .
(a) 0.435 mol Na, 0.217 mol Cl 2 , 15.4 g Cl 2 ; (b) 0.005780 mol HgO, 2.890 10 −3 mol O 2 , 9.248 10 −2 g O 2 ; (c) 8.00 mol NaNO 3 , 6.8 10 2 g NaNO 3 ; (d) 1665 mol CO 2 , 73.3 kg CO 2 ; (e) 18.86 mol CuO, 2.330 kg CuCO 3 ; (f) 0.4580 mol C 2 H 4 Br 2 , 86.05 g C 2 H 4 Br 2
Write the balanced equation, then outline the steps necessary to determine the information requested in each of the following:
(a) The number of moles and the mass of Mg required to react with 5.00 g of HCl and produce MgCl 2 and H 2 .
(b) The number of moles and the mass of oxygen formed by the decomposition of 1.252 g of silver(I) oxide.
(c) The number of moles and the mass of magnesium carbonate, MgCO 3 , required to produce 283 g of carbon dioxide. (MgO is the other product.)
(d) The number of moles and the mass of water formed by the combustion of 20.0 kg of acetylene, C 2 H 2 , in an excess of oxygen.
(e) The number of moles and the mass of barium peroxide, BaO 2 , needed to produce 2.500 kg of barium oxide, BaO (O 2 is the other product.)
(f)


Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?