Number of product molecules generated by a reaction
How many carbon dioxide molecules are produced when 0.75 mol of propane is combusted according to this equation?
Solution
The approach here is the same as for
[link] , though the absolute number of molecules is requested, not the number of moles of molecules. This will simply require use of the moles-to-numbers conversion factor, Avogadro’s number.
The balanced equation shows that carbon dioxide is produced from propane in a 3:1 ratio:
Using this stoichiometric factor, the provided molar amount of propane, and Avogadro’s number,

Check your learning
How many NH
3 molecules are produced by the reaction of 4.0 mol of Ca(OH)
2 according to the following equation:
Answer:
4.8
10
24 NH
3 molecules
Got questions? Get instant answers now!
These examples illustrate the ease with which the amounts of substances involved in a chemical reaction of known stoichiometry may be related. Directly measuring numbers of atoms and molecules is, however, not an easy task, and the practical application of stoichiometry requires that we use the more readily measured property of mass.
Relating masses of reactants and products
What mass of sodium hydroxide, NaOH, would be required to produce 16 g of the antacid milk of magnesia [magnesium hydroxide, Mg(OH)
2 ] by the following reaction?
Solution
The approach used previously in
[link] and
[link] is likewise used here; that is, we must derive an appropriate stoichiometric factor from the balanced chemical equation and use it to relate the amounts of the two substances of interest. In this case, however, masses (not molar amounts) are provided and requested, so additional steps of the sort learned in the previous chapter are required. The calculations required are outlined in this flowchart:
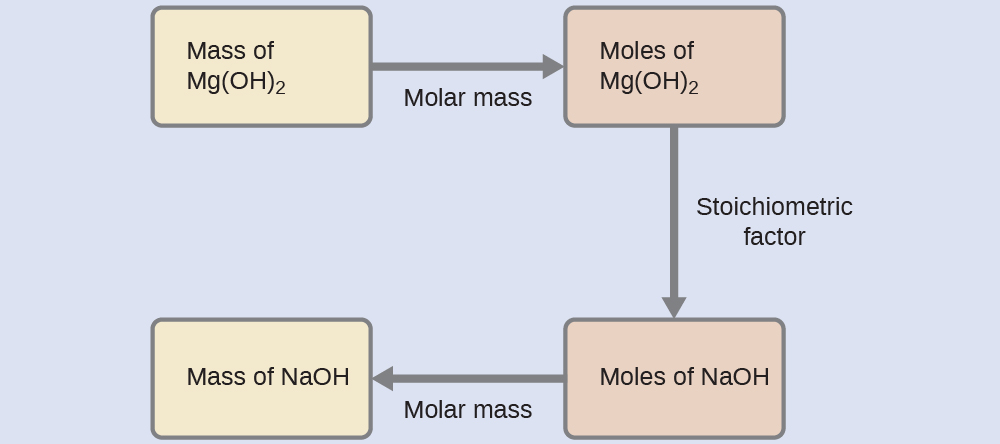
Check your learning
What mass of gallium oxide, Ga
2 O
3 , can be prepared from 29.0 g of gallium metal? The equation for the reaction is
Got questions? Get instant answers now!
Relating masses of reactants
What mass of oxygen gas, O
2 , from the air is consumed in the combustion of 702 g of octane, C
8 H
18 , one of the principal components of gasoline?
Solution
The approach required here is the same as for the
[link] , differing only in that the provided and requested masses are both for reactant species.
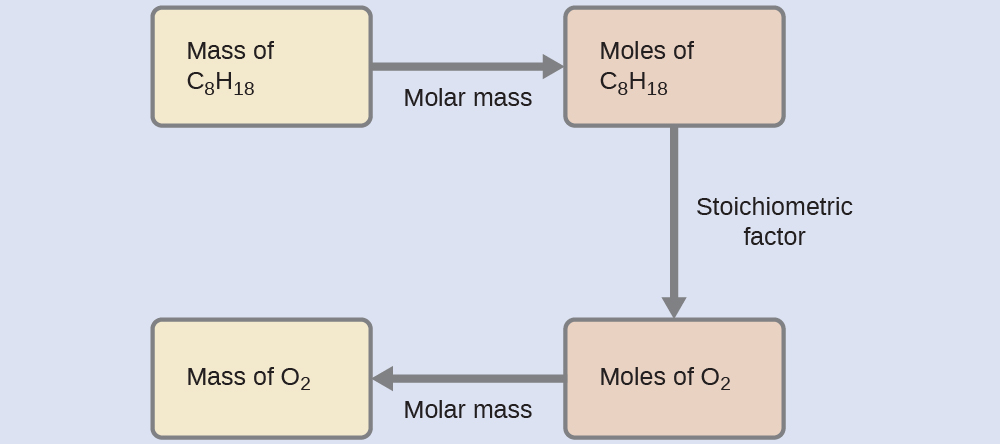
Check your learning
What mass of CO is required to react with 25.13 g of Fe
2 O
3 according to the equation
Got questions? Get instant answers now!
These examples illustrate just a few instances of reaction stoichiometry calculations. Numerous variations on the beginning and ending computational steps are possible depending upon what particular quantities are provided and sought (volumes, solution concentrations, and so forth). Regardless of the details, all these calculations share a common essential component: the use of stoichiometric factors derived from balanced chemical equations.
[link] provides a general outline of the various computational steps associated with many reaction stoichiometry calculations.



