| << Chapter < Page | Chapter >> Page > |
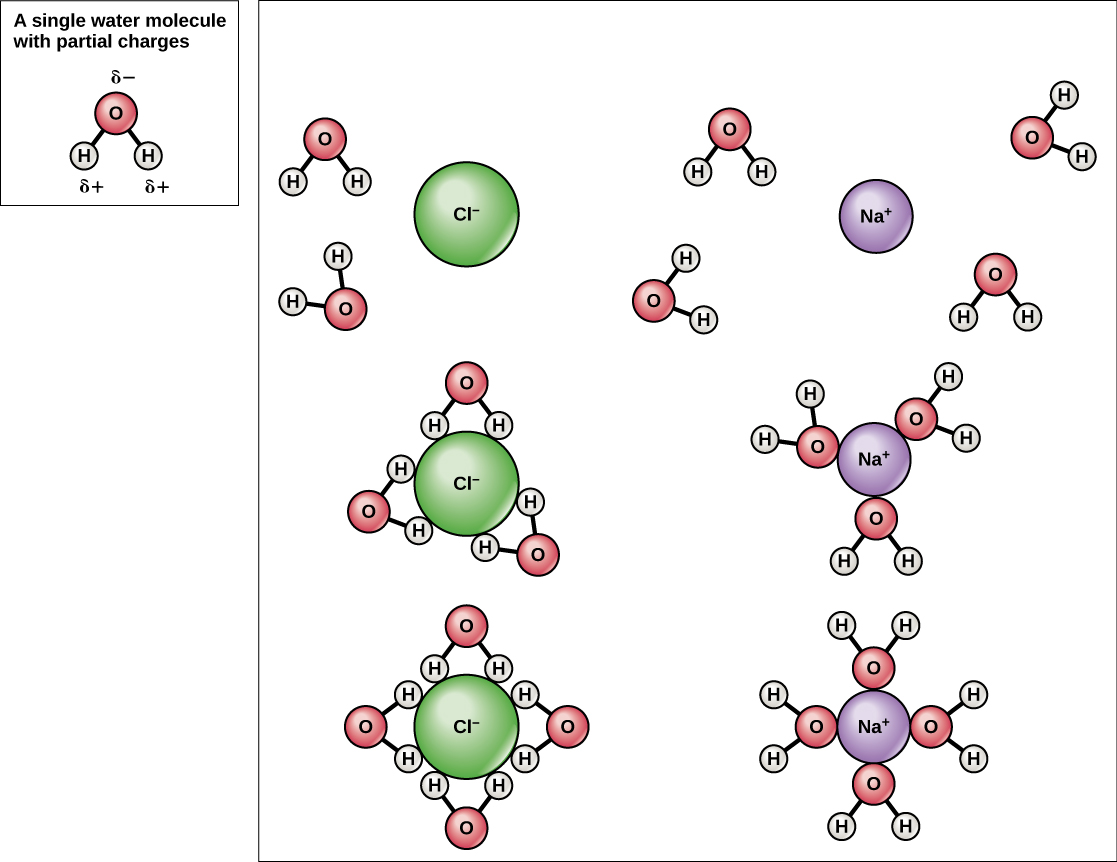
Because water is polar, with slight positive and negative charges, ionic compounds and polar molecules can readily dissolve in it. Water is, therefore, what is referred to as a solvent —a substance capable of dissolving another substance, referred to as the solute, in order to form a solution. The charged particles will form hydrogen bonds with a surrounding layer of water molecules. This is referred to as a sphere of hydration and serves to keep the particles separated or dispersed in the water. In the case of table salt (NaCl) mixed in water ( [link] ), the sodium and chloride ions separate, or dissociate, in the water, and spheres of hydration are formed around the ions. A positively charged sodium ion is surrounded by the partially negative charges of oxygen atoms in water molecules. A negatively charged chloride ion is surrounded by the partially positive charges of hydrogen atoms in water molecules. The polarity of the water molecule makes it an effective solvent and is important in its many roles in living systems.
The pH of a solution is a measure of its acidity or alkalinity. You have probably used litmus paper , paper that has been treated with a natural water-soluble dye so it can be used as a pH indicator, to test how much acid or base (alkalinity) exists in a solution. You might have even used some to make sure the water in an outdoor swimming pool is properly treated. In both cases, this pH test measures the amount of hydrogen ions that exists in a given solution. High concentrations of hydrogen ions yield a low pH, whereas low levels of hydrogen ions result in a high pH. The overall concentration of hydrogen ions is inversely related to its pH and can be measured on the pH scale ( [link] ). Therefore, the more hydrogen ions present, the lower the pH; conversely, the fewer hydrogen ions, the higher the pH.
The pH scale ranges from 0 to 14. A change of one unit on the pH scale represents a change in the concentration of hydrogen ions by a factor of 10, a change in two units represents a change in the concentration of hydrogen ions by a factor of 100. Thus, small changes in pH represent large changes in the concentrations of hydrogen ions. Pure water is neutral. It is neither acidic nor basic, and has a pH of 7.0. Anything below 7.0 (ranging from 0.0 to 6.9) is acidic, and anything above 7.0 (from 7.1 to 14.0) is alkaline. The blood in your veins is slightly alkaline (pH = 7.4). The environment in your stomach is highly acidic (pH = 1 to 2). Orange juice is mildly acidic (pH = approximately 3.5), whereas baking soda is basic (pH = 9.0).

Acids are substances that provide hydrogen ions (H + ) and lower pH, whereas bases provide hydroxide ions (OH – ) and raise pH. The stronger the acid, the more readily it donates H + . For example, hydrochloric acid and lemon juice are very acidic and readily give up H + when added to water. Conversely, bases are those substances that readily donate OH – . The OH – ions combine with H + to produce water, which raises a substance’s pH. Sodium hydroxide and many household cleaners are very alkaline and give up OH – rapidly when placed in water, thereby raising the pH.
Most cells in our bodies operate within a very narrow window of the pH scale, typically ranging only from 7.2 to 7.6. If the pH of the body is outside of this range, the respiratory system malfunctions, as do other organs in the body. Cells no longer function properly, and proteins will break down. Deviation outside of the pH range can induce coma or even cause death.
So how is it that we can ingest or inhale acidic or basic substances and not die? Buffers are the key. Buffers readily absorb excess H + or OH – , keeping the pH of the body carefully maintained in the aforementioned narrow range. Carbon dioxide is part of a prominent buffer system in the human body; it keeps the pH within the proper range. This buffer system involves carbonic acid (H 2 CO 3 ) and bicarbonate (HCO 3 – ) anion. If too much H + enters the body, bicarbonate will combine with the H + to create carbonic acid and limit the decrease in pH. Likewise, if too much OH – is introduced into the system, carbonic acid will rapidly dissociate into bicarbonate and H + ions. The H + ions can combine with the OH – ions, limiting the increase in pH. While carbonic acid is an important product in this reaction, its presence is fleeting because the carbonic acid is released from the body as carbon dioxide gas each time we breathe. Without this buffer system, the pH in our bodies would fluctuate too much and we would fail to survive.
Water has many properties that are critical to maintaining life. It is polar, allowing for the formation of hydrogen bonds, which allow ions and other polar molecules to dissolve in water. Therefore, water is an excellent solvent. The hydrogen bonds between water molecules give water the ability to hold heat better than many other substances. As the temperature rises, the hydrogen bonds between water continually break and reform, allowing for the overall temperature to remain stable, although increased energy is added to the system. All of these unique properties of water are important in the chemistry of living organisms.
The pH of a solution is a measure of the concentration of hydrogen ions in the solution. A solution with a high number of hydrogen ions is acidic and has a low pH value. A solution with a high number of hydroxide ions is basic and has a high pH value. The pH scale ranges from 0 to 14, with a pH of 7 being neutral. Buffers are solutions that moderate pH changes when an acid or base is added to the buffer system. Buffers are important in biological systems because of their ability to maintain constant pH conditions.

Notification Switch
Would you like to follow the 'Human biology' conversation and receive update notifications?