| << Chapter < Page | Chapter >> Page > |
The electron transport chain ( [link] a ) is the last component of aerobic respiration and is the only part of metabolism that uses atmospheric oxygen. Oxygen continuously diffuses into plants for this purpose. In animals, oxygen enters the body through the respiratory system. Electron transport is a series of chemical reactions that resembles a bucket brigade in that electrons are passed rapidly from one component to the next, to the endpoint of the chain where oxygen is the final electron acceptor and water is produced. There are four complexes composed of proteins, labeled I through IV in [link] c , and the aggregation of these four complexes, together with associated mobile, accessory electron carriers, is called the electron transport chain . The electron transport chain is present in multiple copies in the inner mitochondrial membrane of eukaryotes and in the plasma membrane of prokaryotes. In each transfer of an electron through the electron transport chain, the electron loses energy, but with some transfers, the energy is stored as potential energy by using it to pump hydrogen ions across the inner mitochondrial membrane into the intermembrane space, creating an electrochemical gradient.
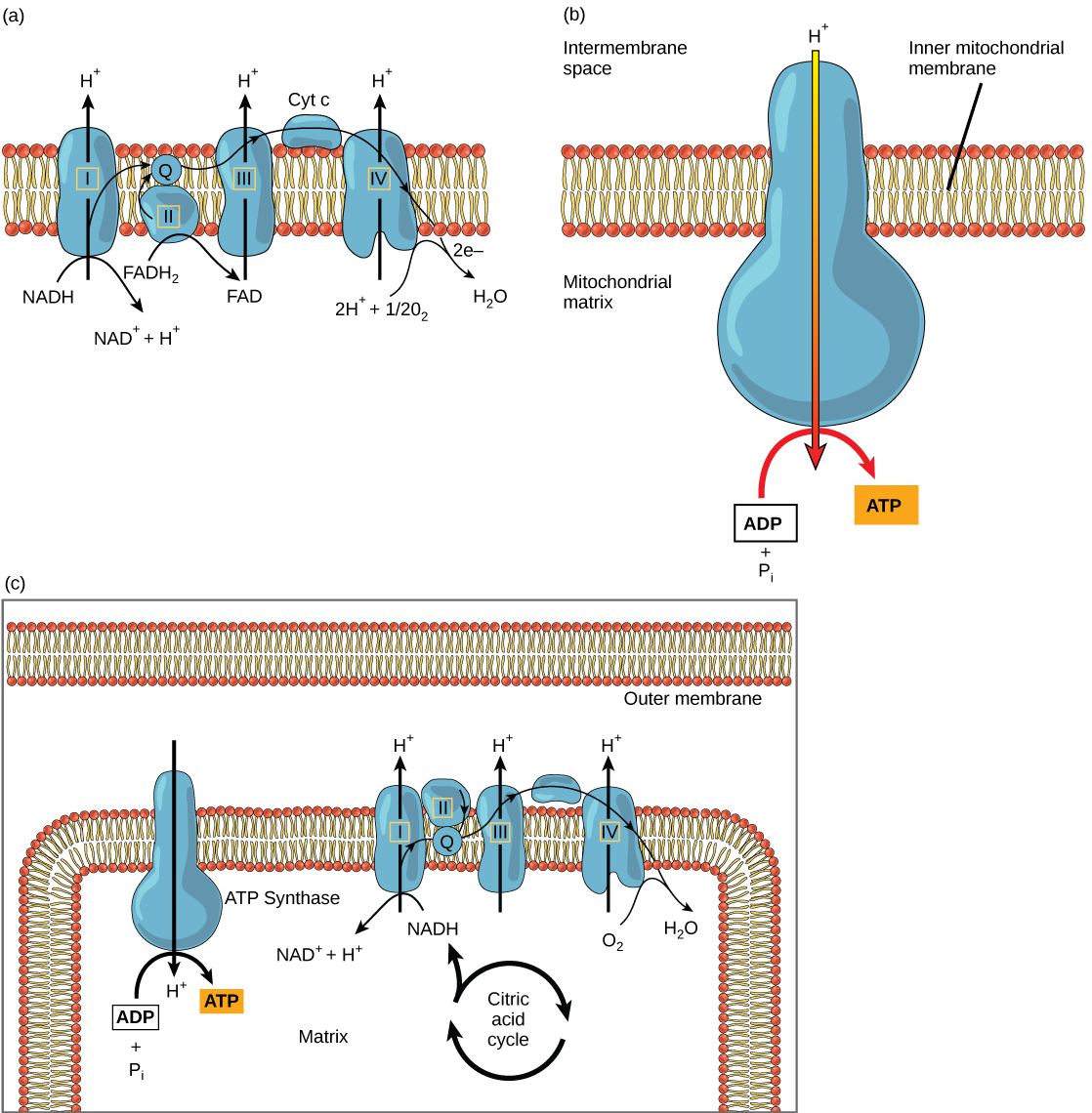
Electrons from NADH and FADH 2 are passed to protein complexes in the electron transport chain. As they are passed from one complex to another (there are a total of four), the electrons lose energy, and some of that energy is used to pump hydrogen ions from the mitochondrial matrix into the intermembrane space. In the fourth protein complex, the electrons are accepted by oxygen, the terminal acceptor. The oxygen with its extra electrons then combines with two hydrogen ions, further enhancing the electrochemical gradient, to form water. If there were no oxygen present in the mitochondrion, the electrons could not be removed from the system, and the entire electron transport chain would back up and stop. The mitochondria would be unable to generate new ATP in this way, and the cell would ultimately die from lack of energy. This is the reason we must breathe to draw in new oxygen.
In the electron transport chain, the free energy from the series of reactions just described is used to pump hydrogen ions (via active transport) across the membrane. The uneven distribution of H + ions across the membrane establishes an electrochemical gradient, owing to the H + ions’ positive charge and their higher concentration on one side of the membrane.
Hydrogen ions diffuse through the inner membrane through a membrane protein called ATP synthase ( [link] b ). This complex protein acts as a tiny generator, turned by the force of the hydrogen ions diffusing through it, down their electrochemical gradient from the intermembrane space, where there are many mutually repelling hydrogen ions to the matrix, where there are few. The turning of the parts of this molecular machine regenerate ATP from ADP and phosphate.
Chemiosmosis ( [link] c ) is used to generate 90 percent of the ATP made during aerobic glucose catabolism. The result of the reactions is the production of ATP from the energy of the electrons removed from hydrogen atoms. These atoms were originally part of a glucose molecule. At the end of the electron transport system, the electrons are used to reduce an oxygen molecule to oxygen ions. The extra electrons on the oxygen ions attract hydrogen ions (protons) from the surrounding medium, and water is formed.
The number of ATP molecules generated from the catabolism of glucose varies. In general, processing of each NADH yields approximately 3 ATP and each FADH 2 yields approximately 2 ATP. Overall, a total of 10 NADH and 2 FADH 2 were produced in glycolysis, transition reaction, and the citric acid cycle per glucose molecule. This results in the production of approximately 34 ATP. Remember, that two additional ATP were produced directly in both glycolysis and the citric acid cycle, resulting in a total yield of 38 ATP per glucose. This represents an efficiency of approximately 35%, with the remaining energy potential lost as heat or other products.
The citric acid cycle is a series of chemical reactions that removes high-energy electrons and uses them in the electron transport chain to generate ATP. One molecule of ATP (or an equivalent) is produced per each turn of the cycle.
The electron transport chain is the portion of aerobic respiration that uses free oxygen as the final electron acceptor for electrons removed from the intermediate compounds in glucose catabolism. The electrons are passed through a series of chemical reactions, with a small amount of free energy used at three points to transport hydrogen ions across the membrane. This contributes to the gradient used in chemiosmosis. As the electrons are passed from NADH or FADH 2 down the electron transport chain, they lose energy. The products of the electron transport chain are water and ATP. A number of intermediate compounds can be diverted into the anabolism of other biochemical molecules, such as nucleic acids, non-essential amino acids, sugars, and lipids. These same molecules, except nucleic acids, can serve as energy sources for the glucose pathway.

Notification Switch
Would you like to follow the 'Human biology' conversation and receive update notifications?