| << Chapter < Page | Chapter >> Page > |
View a simulation on nuclear fission to start a chain reaction, or introduce nonradioactive isotopes to prevent one. Control energy production in a nuclear reactor.
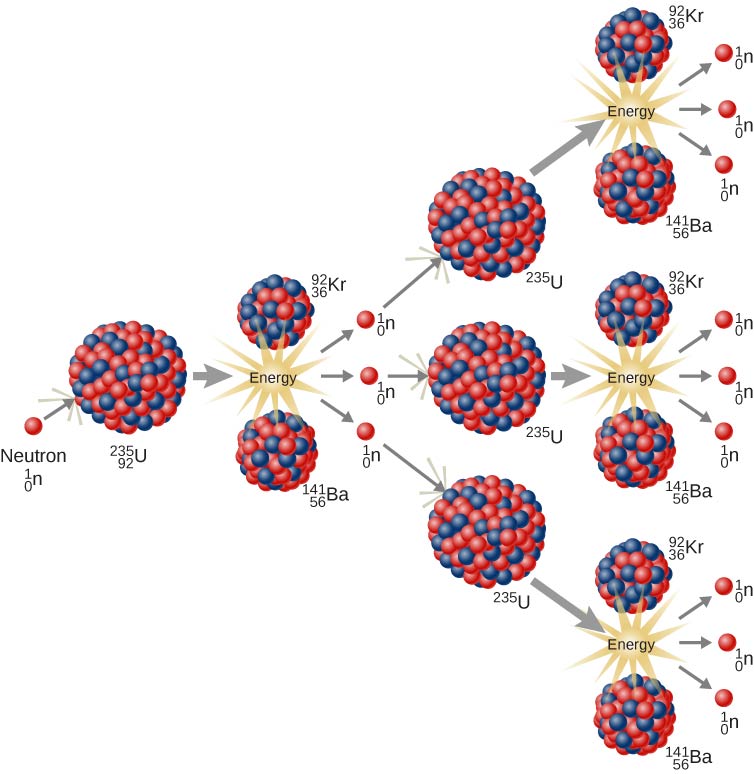
The possibility of a chain reaction in uranium, with its extremely large energy release, led nuclear scientists to conceive of making a bomb—an atomic bomb . (These discoveries were taking place in the years just prior to the Second World War and many of the European physicists involved in these discoveries came from countries that were being overrun.) Natural uranium contains U-238 and only U-235, and does not produce a chain reaction. To produce a controlled, sustainable chain reaction, the percentage of U-235 must be increased to about . In addition, the uranium sample must be massive enough so a typical neutron is more likely to induce fission than it is to escape. The minimum mass needed for the chain reaction to occur is called the critical mass . When the critical mass reaches a point at which the chain reaction becomes self-sustaining, this is a condition known as criticality . The original design required two pieces of U-235 below the critical mass. When one piece in the form of a bullet is fired into the second piece, the critical mass is exceeded and a chain reaction is produced.
An important obstacle to the U-235 bomb is the production of a critical mass of fissionable material. Therefore, scientists developed a plutonium-239 bomb because Pu-239 is more fissionable than U-235 and thus requires a smaller critical mass. The bomb was made in the form of a sphere with pieces of plutonium, each below the critical mass, at the edge of the sphere. A series of chemical explosions fired the plutonium pieces toward the center of the sphere simultaneously. When all these pieces of plutonium came together, the combination exceeded the critical mass and produced a chain reaction. Both the U-235 and Pu-239 bombs were used in World War II. Whether to develop and use atomic weapons remain two of the most important questions faced by human civilization.
The atomic masses are and
The mass lost is the mass of or
Therefore, the energy released is

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?