| << Chapter < Page | Chapter >> Page > |
Fermi energies for selected materials are listed in the following table.
| Element | Conduction Band Electron Density | Free-Electron Model Fermi Energy |
|---|---|---|
Note also that only the graph in part (c) of the figure, which answers the question, “How many particles are found in the energy range?” is checked by experiment. The Fermi temperature or effective “temperature” of an electron at the Fermi energy is
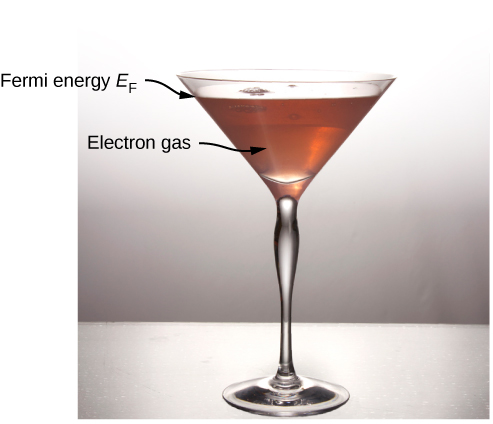
To visualize how the quantum states are filled, we might imagine pouring water slowly into a glass, such as that of [link] . The first drops of water (the electrons) occupy the bottom of the glass (the states with lowest energy). As the level rises, states of higher and higher energy are occupied. Furthermore, since the glass has a wide opening and a narrow stem, more water occupies the top of the glass than the bottom. This reflects the fact that the density of states g ( E ) is proportional to , so there is a relatively large number of higher energy electrons in a free electron gas. Finally, the level to which the glass is filled corresponds to the Fermi energy.
Suppose that at , the number of conduction electrons per unit volume in our sample is . Since each field state has one electron, the number of filled states per unit volume is the same as the number of electrons per unit volume.
Why does the Fermi energy increase with the number of electrons in a metal?
If the electron number density ( N/V ) of a metal increases by a factor 8, what happens to the Fermi energy
increases by a factor of
Why does the horizontal line in the graph in [link] suddenly stop at the Fermi energy?
Why does the graph in [link] increase gradually from the origin?
For larger energies, the number of accessible states increases.
Why are the sharp transitions at the Fermi energy “smoothed out” by increasing the temperature?
What is the difference in energy between the state and the state with the next higher energy? What is the percentage change in the energy between the state and the state with the next higher energy? (b) Compare these with the difference in energy and the percentage change in the energy between the state and the state with the next higher energy.
a. ; b. ; for very large values of the quantum numbers, the spacing between adjacent energy levels is very small (“in the continuum”). This is consistent with the expectation that for large quantum numbers, quantum and classical mechanics give approximately the same predictions.
An electron is confined to a metal cube of on each side. Determine the density of states at (a) ; (b) ; and (c) .
What value of energy corresponds to a density of states of ?
10.0 eV
Compare the density of states at 2.5 eV and 0.25 eV.
Consider a cube of copper with edges 1.50 mm long. Estimate the number of electron quantum states in this cube whose energies are in the range 3.75 to 3.77 eV.
If there is one free electron per atom of copper, what is the electron number density of this metal?
Determine the Fermi energy and temperature for copper at .
Fermi energy, Temperature,

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?