| << Chapter < Page | Chapter >> Page > |

The study of atomic energy transitions enables us to understand X-rays and X-ray technology. Like all electromagnetic radiation, X-rays are made of photons. X-ray photons are produced when electrons in the outermost shells of an atom drop to the inner shells. (Hydrogen atoms do not emit X-rays, because the electron energy levels are too closely spaced together to permit the emission of high-frequency radiation.) Transitions of this kind are normally forbidden because the lower states are already filled. However, if an inner shell has a vacancy (an inner electron is missing, perhaps from being knocked away by a high-speed electron), an electron from one of the outer shells can drop in energy to fill the vacancy. The energy gap for such a transition is relatively large, so wavelength of the radiated X-ray photon is relatively short.
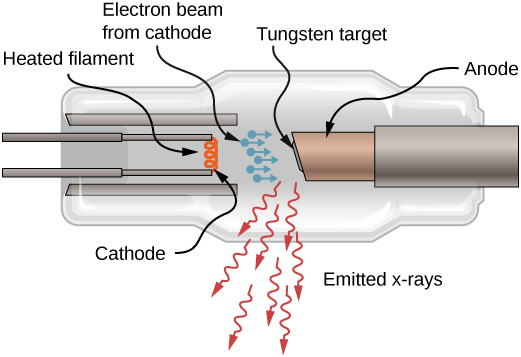
X-rays can also be produced by bombarding a metal target with high-energy electrons, as shown in [link] . In the figure, electrons are boiled off a filament and accelerated by an electric field into a tungsten target. According to the classical theory of electromagnetism, any charged particle that accelerates emits radiation. Thus, when the electron strikes the tungsten target, and suddenly slows down, the electron emits braking radiation . (Braking radiation refers to radiation produced by any charged particle that is slowed by a medium.) In this case, braking radiation contains a continuous range of frequencies, because the electrons will collide with the target atoms in slightly different ways.
Braking radiation is not the only type of radiation produced in this interaction. In some cases, an electron collides with another inner-shell electron of a target atom, and knocks the electron out of the atom—billiard ball style. The empty state is filled when an electron in a higher shell drops into the state (drop in energy level) and emits an X-ray photon.
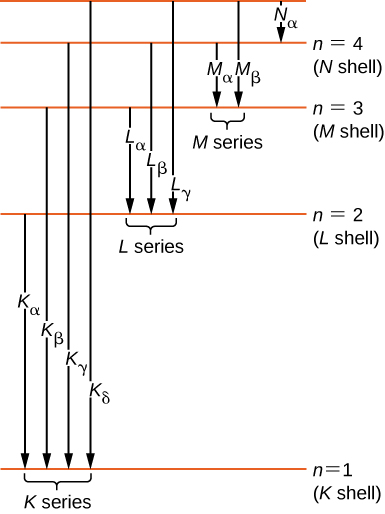
Historically, X-ray spectral lines were labeled with letters ( K , L , M , N , …). These letters correspond to the atomic shells ( ). X-rays produced by a transition from any higher shell to the K ( ) shell are labeled as K X-rays. X-rays produced in a transition from the L ( ) shell are called X-rays; X-rays produced in a transition from the M ( ) shell are called X-rays; X-rays produced in a transition from the N ( ) shell are called X-rays; and so forth. Transitions from higher shells to L and M shells are labeled similarly. These transitions are represented by an energy-level diagram in [link] .
The distribution of X-ray wavelengths produced by striking metal with a beam of electrons is given in [link] . X-ray transitions in the target metal appear as peaks on top of the braking radiation curve. Photon frequencies corresponding to the spikes in the X-ray distribution are called characteristic frequencies, because they can be used to identify the target metal. The sharp cutoff wavelength (just below the peak) corresponds to an electron that loses all of its energy to a single photon. Radiation of shorter wavelengths is forbidden by the conservation of energy.

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?