| << Chapter < Page | Chapter >> Page > |
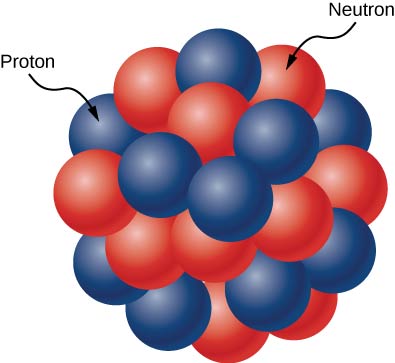
The atomic nucleus is composed of protons and neutrons ( [link] ). Protons and neutrons have approximately the same mass, but protons carry one unit of positive charge and neutrons carry no charge. These particles are packed together into an extremely small space at the center of an atom. According to scattering experiments, the nucleus is spherical or ellipsoidal in shape, and about 1/100,000th the size of a hydrogen atom. If an atom were the size of a major league baseball stadium, the nucleus would be roughly the size of the baseball. Protons and neutrons within the nucleus are called nucleons .
The number of protons in the nucleus is given by the atomic number , Z . The number of neutrons in the nucleus is the neutron number , N . The total number of nucleons is the mass number , A . These numbers are related by
A nucleus is represented symbolically by
where X represents the chemical element, A is the mass number, and Z is the atomic number. For example, represents the carbon nucleus with six protons and six neutrons (or 12 nucleons).
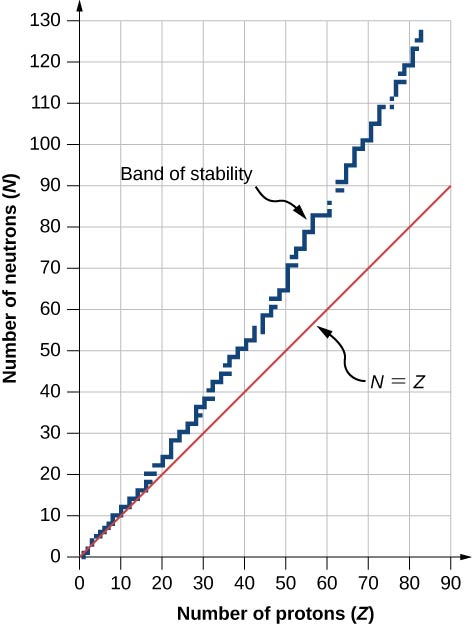
A graph of the number N of neutrons versus the number Z of protons for a range of stable nuclei ( nuclide s ) is shown in [link] . For a given value of Z , multiple values of N (blue points) are possible. For small values of Z , the number of neutrons equals the number of protons and the data fall on the red line. For large values of Z, the number of neutrons is greater than the number of protons and the data points fall above the red line. The number of neutrons is generally greater than the number of protons for
A chart based on this graph that provides more detailed information about each nucleus is given in [link] . This chart is called a chart of the nuclides . Each cell or tile represents a separate nucleus. The nuclei are arranged in order of ascending Z (along the horizontal direction) and ascending N (along the vertical direction).
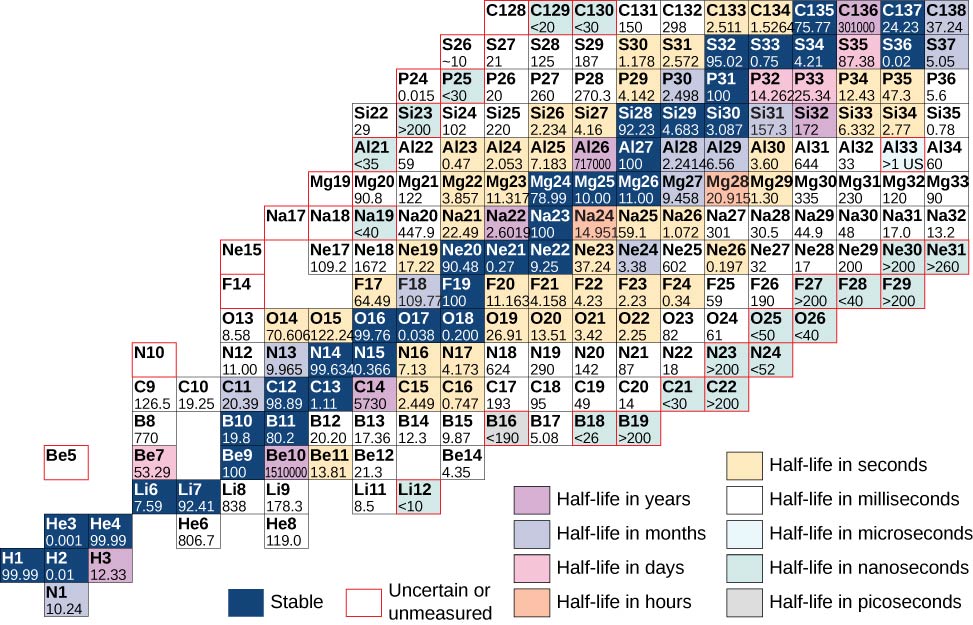
Atoms that contain nuclei with the same number of protons ( Z ) and different numbers of neutrons ( N ) are called isotopes . For example, hydrogen has three isotopes: normal hydrogen (1 proton, no neutrons), deuterium (one proton and one neutron), and tritium (one proton and two neutrons). Isotopes of a given atom share the same chemical properties, since these properties are determined by interactions between the outer electrons of the atom, and not the nucleons. For example, water that contains deuterium rather than hydrogen (“heavy water”) looks and tastes like normal water. The following table shows a list of common isotopes.

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?