| << Chapter < Page | Chapter >> Page > |
Experiments with electric charges have shown that if two objects each have electric charge, then they exert an electric force on each other. The magnitude of the force is linearly proportional to the net charge on each object and inversely proportional to the square of the distance between them. (Interestingly, the force does not depend on the mass of the objects.) The direction of the force vector is along the imaginary line joining the two objects and is dictated by the signs of the charges involved.
Let
The electric force on one of the charges is proportional to the magnitude of its own charge and the magnitude of the other charge, and is inversely proportional to the square of the distance between them:
This proportionality becomes an equality with the introduction of a proportionality constant. For reasons that will become clear in a later chapter, the proportionality constant that we use is actually a collection of constants. (We discuss this constant shortly.)
The electric force (or Coulomb force ) between two electrically charged particles is equal to
We use absolute value signs around the product because one of the charges may be negative, but the magnitude of the force is always positive. The unit vector points directly from the charge toward . If and have the same sign, the force vector on points away from ; if they have opposite signs, the force on points toward ( [link] ).
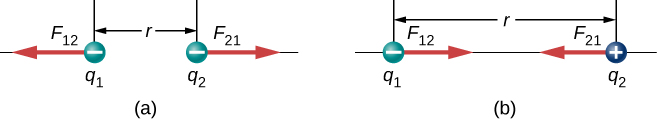
It is important to note that the electric force is not constant; it is a function of the separation distance between the two charges. If either the test charge or the source charge (or both) move, then changes, and therefore so does the force. An immediate consequence of this is that direct application of Newton’s laws with this force can be mathematically difficult, depending on the specific problem at hand. It can (usually) be done, but we almost always look for easier methods of calculating whatever physical quantity we are interested in. (Conservation of energy is the most common choice.)
Finally, the new constant in Coulomb’s law is called the permittivity of free space , or (better) the permittivity of vacuum . It has a very important physical meaning that we will discuss in a later chapter; for now, it is simply an empirical proportionality constant. Its numerical value (to three significant figures) turns out to be

Notification Switch
Would you like to follow the 'University physics volume 2' conversation and receive update notifications?