| << Chapter < Page | Chapter >> Page > |
This mystery was resolved in 1932 by the English physicist James Chadwick , with the discovery of the neutron . The neutron is, essentially, an electrically neutral twin of the proton, with no electric charge, but (nearly) identical mass to the proton. The helium nucleus therefore has two neutrons along with its two protons. (Later experiments were to show that although the neutron is electrically neutral overall, it does have an internal charge structure . Furthermore, although the masses of the neutron and the proton are nearly equal, they aren’t exactly equal: The neutron’s mass is very slightly larger than the mass of the proton. That slight mass excess turned out to be of great importance. That, however, is a story that will have to wait until our study of modern physics in Nuclear Physics .)
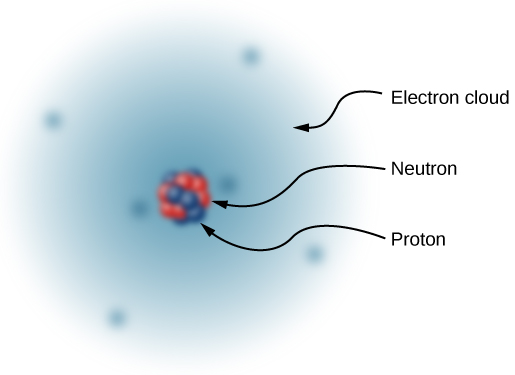
Thus, in 1932, the picture of the atom was of a small, massive nucleus constructed of a combination of protons and neutrons, surrounded by a collection of electrons whose combined motion formed a sort of negatively charged “cloud” around the nucleus ( [link] ). In an electrically neutral atom, the total negative charge of the collection of electrons is equal to the total positive charge in the nucleus. The very low-mass electrons can be more or less easily removed or added to an atom, changing the net charge on the atom (though without changing its type). An atom that has had the charge altered in this way is called an ion . Positive ions have had electrons removed, whereas negative ions have had excess electrons added. We also use this term to describe molecules that are not electrically neutral.
The story of the atom does not stop there, however. In the latter part of the twentieth century, many more subatomic particles were discovered in the nucleus of the atom: pions, neutrinos, and quarks, among others. With the exception of the photon, none of these particles are directly relevant to the study of electromagnetism, so we defer further discussion of them until the chapter on particle physics ( Particle Physics and Cosmology ).
As noted previously, electric charge is a property that an object can have. This is similar to how an object can have a property that we call mass, a property that we call density, a property that we call temperature, and so on. Technically, we should always say something like, “Suppose we have a particle that carries a charge of ” However, it is very common to say instead, “Suppose we have a charge.” Similarly, we often say something like, “Six charges are located at the vertices of a regular hexagon.” A charge is not a particle; rather, it is a property of a particle. Nevertheless, this terminology is extremely common (and is frequently used in this book, as it is everywhere else). So, keep in the back of your mind what we really mean when we refer to a “charge.”
There are very large numbers of charged particles in most objects. Why, then, don’t most objects exhibit static electricity?
There are mostly equal numbers of positive and negative charges present, making the object electrically neutral.
Why do most objects tend to contain nearly equal numbers of positive and negative charges?
A positively charged rod attracts a small piece of cork. (a) Can we conclude that the cork is negatively charged? (b) The rod repels another small piece of cork. Can we conclude that this piece is positively charged?
a. yes; b. yes
Two bodies attract each other electrically. Do they both have to be charged? Answer the same question if the bodies repel one another.
How would you determine whether the charge on a particular rod is positive or negative?
Take an object with a known charge, either positive or negative, and bring it close to the rod. If the known charged object is positive and it is repelled from the rod, the rod is charged positive. If the positively charged object is attracted to the rod, the rod is negatively charged.
Common static electricity involves charges ranging from nanocoulombs to microcoulombs. (a) How many electrons are needed to form a charge of −2.00 nC? (b) How many electrons must be removed from a neutral object to leave a net charge of ?
a.
;
b.
If electrons move through a pocket calculator during a full day’s operation, how many coulombs of charge moved through it?
To start a car engine, the car battery moves electrons through the starter motor. How many coulombs of charge were moved?
A certain lightning bolt moves 40.0 C of charge. How many fundamental units of charge is this?
A 2.5-g copper penny is given a charge of . (a) How many excess electrons are on the penny? (b) By what percent do the excess electrons change the mass of the penny?
a.
;
b.
A 2.5-g copper penny is given a charge of . (a) How many electrons are removed from the penny? (b) If no more than one electron is removed from an atom, what percent of the atoms are ionized by this charging process?

Notification Switch
Would you like to follow the 'University physics volume 2' conversation and receive update notifications?