| << Chapter < Page | Chapter >> Page > |
Electrochemistry has a number of different uses, particularly in industry. We are going to look at a few examples.
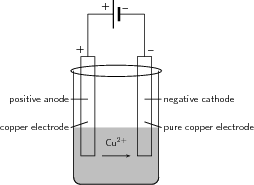
Electroplating is the process of using electrical current to coat an electrically conductive object with a thin layer of metal. Mostly, this application is used to deposit a layer of metal that has some desired property (e.g. abrasion and wear resistance, corrosion protection, improvement of aesthetic qualities etc.) onto a surface that doesn't have that property. Electro-refining (also sometimes called electrowinning is electroplating on a large scale. Electrochemical reactions are used to deposit pure metals from their ores. One example is the electrorefining of copper.
Copper plays a major role in the electrical reticulation industry as it is very conductive and is used in electric cables. One of the problems though is that copper must be pure if it is to be an effective current carrier. One of the methods used to purify copper, is electrowinning. The copper electrowinning process is as follows:
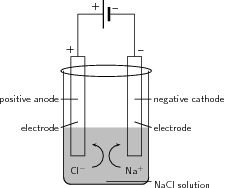
Electrolysis can also be used to produce chlorine gas from brine/seawater (NaCl). This is sometimes referred to as the 'Chlor-alkali' process. The reactions that take place are as follows:
At the anode the reaction is:
whereas at the cathode , the following happens:
The overall reaction is:
Chlorine is a very important chemical. It is used as a bleaching agent, a disinfectant, in solvents, pharmaceuticals, dyes and even plastics such as polyvinlychloride (PVC).
Aluminum metal is a commonly used metal in industry where its properties of being both light and strong can be utilized. It is also used in the manufacture of products such as aeroplanes and motor cars. The metal is present in deposits of bauxite which is a mixture of silicas, iron oxides and hydrated alumina ( x ).
Electrolysis can be used to extract aluminum from bauxite. The process described below produces pure aluminum:

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 12 physical science' conversation and receive update notifications?